Characteristics of a Novel ATP2B3 K416_F418delinsN Mutation in a Classical Aldosterone-Producing Adenoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Diagnosis of PA
2.3. Genomic DNA Extraction
2.4. ATP2B3 Gene Sequencing
2.5. Immunohistochemistry of Resected Tissues
2.6. Culture of Cell Line
2.7. Plasmid and Transfection
2.8. Western Blot Analysis
2.9. Analysis of Aldosterone
2.10. Statistical Analysis
3. Results
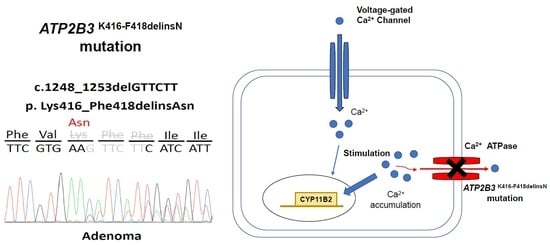
3.1. Identifying the ATP2B3 K416_F418delinsN Gene and Demographics of the Specific Patient
3.2. The Immunochemistry Staining of CYP11B2 on Excised Adrenal Tissue
3.3. The Aldosterone Synthase of ATP2B3 K416-F418delinsN Mutation
4. Discussion
4.1. Calcium Channel and Somatic Mutations in APA
4.2. Mutant ATP2B3 and APA
4.3. Bilateral Asymmetric Manifestations of the APA
4.4. Clinical Implication and Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omata, K.; Satoh, F.; Morimoto, R.; Ito, S.; Yamazaki, Y.; Nakamura, Y.; Anand, S.K.; Guo, Z.; Stowasser, M.; Sasano, H.; et al. Cellular and Genetic Causes of Idiopathic Hyperaldosteronism. Hypertension 2018, 72, 874–880. [Google Scholar] [CrossRef]
- Young, W.F. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2019, 285, 126–148. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.-C.; Wu, V.-C.; Huang, K.-H.; Wang, S.-M.; Chang, C.-C.; Lu, C.-C.; Yang, W.-S.; Tsai, C.-W.; Lai, C.-F.; Lee, T.-Y.; et al. Verification and evaluation of aldosteronism demographics in the Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group). J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 348–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.-C.; Chueh, S.; Chang, H.; Lin, W.-C.; Liu, K.-L.; Li, H.; Lin, Y.-H.; Wu, K.-D.; Hsieh, B. Bilateral aldosterone-producing adenomas: Differentiation from bilateral adrenal hyperplasia. Qjm 2007, 101, 13–22. [Google Scholar] [CrossRef]
- Choi, M.; Scholl, U.I.; Yue, P.; Björklund, P.; Zhao, B.; Nelson-Williams, C.; Ji, W.; Cho, Y.; Patel, A.; Men, C.J.; et al. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science 2011, 331, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, U.I.; Goh, G.; Stölting, G.; De Oliveira, R.C.; Choi, M.; Overton, J.D.; Fonseca, A.L.; Korah, R.; Starker, L.F.; Kunstman, J.; et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 2013, 45, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Daniil, G.; Fernandes-Rosa, F.L.; Chemin, J.; Blesneac, I.; Beltrand, J.; Polak, M.; Jeunemaitre, X.; Boulkroun, S.; Amar, L.; Strom, T.M.; et al. CACNA1H Mutations Are Associated with Different Forms of Primary Aldosteronism. EBioMedicine 2016, 13, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Rosa, F.L.; Daniil, G.; Orozco, I.J.; Göppner, C.; El Zein, R.; Jain, V.; Boulkroun, S.; Jeunemaitre, X.; Amar, L.; Lefebvre, H.; et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat. Genet. 2018, 50, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Beuschlein, F.; Boulkroun, S.; Osswald, A.; Wieland, T.; Nielsen, H.N.; Lichtenauer, U.D.; Penton, D.; Schack, V.R.; Amar, L.; Fischer, E.; et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 2013, 45, 441–442. [Google Scholar] [CrossRef]
- Seccia, T.M.; Caroccia, B.; Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E.; Rossi, G.P. The Biology of Normal Zona Glomerulosa And Aldosterone-Producing Adenoma: Pathological Implications. Endocr. Rev. 2018, 39, 1029–1056. [Google Scholar] [CrossRef] [Green Version]
- Tauber, P.; Aichinger, B.; Christ, C.; Stindl, J.; Rhayem, Y.; Beuschlein, F.; Warth, R.; Bandulik, S. Cellular Pathophysiology of an Adrenal Adenoma-Associated Mutant of the Plasma Membrane Ca2+-ATPase ATP2B3. Endocrinology 2016, 157, 2489–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Rosa, F.L.; Boulkroun, S.; Zennaro, M.-C. Genetic and Genomic Mechanisms of Primary Aldosteronism. Trends Mol. Med. 2020, 26, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Monticone, S.; Schack, V.R.; Stindl, J.; Burrello, J.; Buffolo, F.; Annaratone, L.; Castellano, I.; Beuschlein, F.; Reincke, M.; et al. Somatic ATP1A1, ATP2B3, and KCNJ5 Mutations in Aldosterone-Producing Adenomas. Hypertension 2014, 63, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.-C.; Wang, S.-M.; Chueh, S.-C.J.; Yang, S.-Y.; Huang, K.-H.; Lin, Y.-H.; Wang, J.-J.; Connolly, R.; Hu, Y.-H.; Gomez-Sanchez, C.E.; et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci. Rep. 2017, 7, 39121. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Wu, V.-C.; Kuo, C.-C.; Lin, Y.-H.; Chang, C.-C.; Chueh, S.J.; Wu, K.-D.; Pimenta, E.; Stowasser, M. Diagnosis and management of primary aldosteronism: An updated review. Ann. Med. 2013, 45, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Hu, Y.-H.; Er, L.K.; Yen, R.-F.; Chang, C.-H.; Chang, Y.-L.; Lu, C.-C.; Chang, C.-C.; Lin, J.-H.; Lin, Y.-H.; et al. Case detection and diagnosis of primary aldosteronism—The consensus of Taiwan Society of Aldosteronism. J. Formos. Med. Assoc. 2017, 116, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-Y.; Chang, H.-M.; Lin, Y.-F.; Chan, C.-K.; Chang, C.-H.; Chueh, S.-C.J.; Yang, S.-Y.; Huang, K.-H.; Lin, Y.-H.; Wu, V.-C.; et al. miRNA-203 Modulates Aldosterone Levels and Cell Proliferation by Targeting Wnt5a in Aldosterone-Producing Adenomas. J. Clin. Endocrinol. Metab. 2018, 103, 3737–3747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, K.-Y.; Liao, H.-W.; Chan, C.-K.; Lin, W.-C.; Yang, S.-Y.; Tsai, Y.-C.; Huang, K.-H.; Lin, Y.-H.; Chueh, J.S.; Wu, V.-C. Presence of Subclinical Hypercortisolism in Clinical Aldosterone-Producing Adenomas Predicts Lower Clinical Success. Hypertension 2020, 76, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Kuo, C.-C.; Wang, S.-M.; Liu, K.-L.; Huang, K.-H.; Lin, Y.-H.; Chu, T.-S.; Chang, H.-W.; Lin, C.-Y.; Tsai, C.-T.; et al. Primary aldosteronism: Changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J. Hypertens. 2011, 29, 1778–1786. [Google Scholar] [CrossRef]
- Wu, V.-C.; Lo, S.-C.; Chen, Y.-L.; Huang, P.-H.; Tsai, C.-T.; Liang, C.-J.; Kuo, C.-C.; Kuo, Y.-S.; Lee, B.-C.; Wu, E.-L.; et al. Endothelial Progenitor Cells in Primary Aldosteronism: A Biomarker of Severity for Aldosterone Vasculopathy and Prognosis. J. Clin. Endocrinol. Metab. 2011, 96, 3175–3183. [Google Scholar] [CrossRef] [Green Version]
- Wu, V.-C.; Huang, K.-H.; Peng, K.-Y.; Tsai, Y.-C.; Wu, C.-H.; Wang, S.-M.; Yang, S.-Y.; Lin, L.-Y.; Chang, C.-C.; Lin, Y.-H.; et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci. Rep. 2015, 5, 11396. [Google Scholar] [CrossRef]
- Wu, V.-C.; Chueh, S.-C.; Chang, H.-W.; Lin, L.-Y.; Liu, K.-L.; Lin, Y.-H.; Ho, Y.-L.; Lin, W.-C.; Wang, S.-M.; Huang, K.-H.; et al. Association of Kidney Function With Residual Hypertension After Treatment of Aldosterone-Producing Adenoma. Am. J. Kidney Dis. 2009, 54, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Yang, S.-Y.; Lin, J.-W.; Cheng, B.-W.; Kuo, C.-C.; Tsai, C.-T.; Chu, T.-S.; Huang, K.-H.; Wang, S.-M.; Lin, Y.-H.; et al. Kidney impairment in primary aldosteronism. Clin. Chim. Acta 2011, 412, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.-C.; Chang, H.-W.; Liu, K.-L.; Lin, Y.-H.; Chueh, S.-C.; Lin, W.-C.; Ho, Y.-L.; Huang, J.-W.; Chiang, C.-K.; Yang, S.-Y.; et al. Primary Aldosteronism: Diagnostic Accuracy of the Losartan and Captopril Tests. Am. J. Hypertens. 2009, 22, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, V.-C.; Wang, S.-M.; Chang, C.-H.; Hu, Y.-H.; Lin, L.-Y.; Lin, Y.-H.; Chueh, S.-C.J.; Chen, L.; Wu, K.-D. Long term outcome of Aldosteronism after target treatments. Sci. Rep. 2016, 6, 32103. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-K.; Yang, W.-S.; Lin, Y.-H.; Huang, K.-H.; Lu, C.-C.; Hu, Y.-H.; Wu, V.-C.; Chueh, J.S.; Chu, T.-S.; Chen, Y.-M. Arterial Stiffness is Associated with Clinical Outcome and Cardiorenal Injury in Lateralized Primary Aldosteronism. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E.; Qi, X.; Velarde-Miranda, C.; Plonczynski, M.W.; Parker, C.R.; Rainey, W.; Satoh, F.; Maekawa, T.; Nakamura, Y.; Sasano, H.; et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol. Cell. Endocrinol. 2014, 383, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.-Y.; Liao, H.-W.; Chueh, J.S.; Pan, C.-Y.; Lin, Y.-H.; Chen, Y.-M.; Chen, P.-Y.; Huang, C.-L.; Wu, V.-C. Pathophysiological and Pharmacological Characteristics of KCNJ5 157-159delITE Somatic Mutation in Aldosterone-Producing Adenomas. Biomedicines 2021, 9, 1026. [Google Scholar] [CrossRef]
- Monticone, S.; Hattangady, N.G.; Penton, D.; Isales, C.M.; Edwards, M.A.; Williams, T.A.; Sterner, C.; Warth, R.; Mulatero, P.; Rainey, W.E. a Novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J. Clin. Endocrinol. Metab. 2013, 98, E1861–E1865. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Omasits, U.; Ahrens, C.; Müller, S.; Wollscheid, B. Protter: Interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protter Software Application. Available online: http://wlab.ethz.ch/protter/start/ (accessed on 22 July 2021).
- Nanba, K.; Chen, A.; Nishimoto, K.; Rainey, W.E. Role of Ca2+/Calmodulin-Dependent Protein Kinase Kinase in Adrenal Aldosterone Production. Endocrinology 2015, 156, 1750–1756. [Google Scholar] [CrossRef]
- Seidel, E.; Schewe, J.; Scholl, U.I. Genetic causes of primary aldosteronism. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Rosa, F.L.; Boulkroun, S.; Zennaro, M.-C. Somatic and inherited mutations in primary aldosteronism. J. Mol. Endocrinol. 2017, 59, R47–R63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes-Rosa, F.L.; Williams, T.A.; Riester, A.; Steichen, O.; Beuschlein, F.; Boulkroun, S.; Strom, T.M.; Monticone, S.; Amar, L.; Meatchi, T.; et al. Genetic Spectrum and Clinical Correlates of Somatic Mutations in Aldosterone-Producing Adenoma. Hypertension 2014, 64, 354–361. [Google Scholar] [CrossRef]
- Itcho, K.; Oki, K.; Ohno, H.; Yoneda, M. Update on Genetics of Primary Aldosteronism. Biomedicines 2021, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Auchus, R.J.; Brown, M.; Lenders, J.W.; Naruse, M.; Plouin, P.F.; Satoh, F.; Young, W.F. An Expert Consensus Statement on Use of Adrenal Vein Sampling for the Subtyping of Primary Aldosteronism. Hypertension 2014, 63, 151–160. [Google Scholar] [CrossRef]
- Kline, G.; Chin, A.; So, B.; Harvey, A.; Pasieka, J. Defining contralateral adrenal suppression in primary aldosteronism: Implications for diagnosis and outcome. Clin. Endocrinol. 2015, 83, 20–27. [Google Scholar] [CrossRef]
- Lee, J.; Kang, B.; Ha, J.; Kim, M.-H.; Choi, B.; Hong, T.-H.; Kang, M.I.; Lim, D.-J. Clinical outcomes of primary aldosteronism based on lateralization index and contralateral suppression index after adrenal venous sampling in real-world practice: A retrospective cohort study. BMC Endocr. Disord. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]



| Variables | ATP2B3 K416_F418delinsN Mutation |
|---|---|
| Age (years old) | 53 |
| Sex | male |
| Body weight (kg) | 75 |
| BMI (kg/m2) | 25.95 |
| CT mass size (cm) | Left: 1.6; Right: 0.5 |
| AVS (aldosterone, ng/dL)/cortisol (μg/dL) | |
| CLS | 0.12 |
| LI | 2.2 |
| NP-59 | Bilateral adrenal gland hyperfunction with left side predominance |
| Hypertension duration (years) | 4 |
| SBP (mm Hg) | 197 |
| SBP 12 mon | 158 |
| DBP (mm Hg) | 92 |
| DBP 12 mon | 88 |
| Aldosterone level (ng/dL) † | 59.3 |
| PRA (ng/mL/hr) † | 0.55 |
| ARR(ng/dL per ng/mL/h) | 107.82 |
| K (mEq/L) † | 2.8 |
| At 12 months after adrenalectomy | |
| Aldosterone level | 30.5 |
| PRA | 0.09 |
| K (mEq/L) † | 4.0 |
| ARR (ng/dL per ng/mL/h) | 338.33 |
| Clinical success | partial success § |
| Biochemical success | partial success |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, H.-W.; Peng, K.-Y.; Wu, V.-C.; Lin, Y.-H.; Lin, S.-L.; Lin, W.-C.; Chueh, J.S.; on behalf of (TAIPAI) Study Group. Characteristics of a Novel ATP2B3 K416_F418delinsN Mutation in a Classical Aldosterone-Producing Adenoma. Cancers 2021, 13, 4729. https://doi.org/10.3390/cancers13184729
Liao H-W, Peng K-Y, Wu V-C, Lin Y-H, Lin S-L, Lin W-C, Chueh JS, on behalf of (TAIPAI) Study Group. Characteristics of a Novel ATP2B3 K416_F418delinsN Mutation in a Classical Aldosterone-Producing Adenoma. Cancers. 2021; 13(18):4729. https://doi.org/10.3390/cancers13184729
Chicago/Turabian StyleLiao, Hung-Wei, Kang-Yung Peng, Vin-Cent Wu, Yen-Hung Lin, Shuei-Liong Lin, Wei-Chou Lin, Jeff S. Chueh, and on behalf of (TAIPAI) Study Group. 2021. "Characteristics of a Novel ATP2B3 K416_F418delinsN Mutation in a Classical Aldosterone-Producing Adenoma" Cancers 13, no. 18: 4729. https://doi.org/10.3390/cancers13184729