Enhanced Paclitaxel Efficacy to Suppress Triple-Negative Breast Cancer Progression Using Metronomic Chemotherapy with a Controlled Release System of Electrospun Poly-d-l-Lactide-Co-Glycolide (PLGA) Nanofibers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
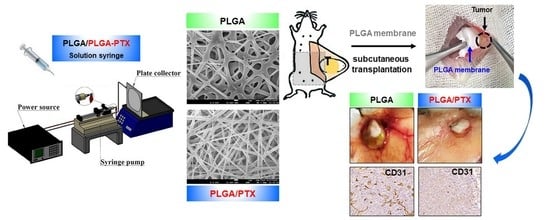
2.2. Preparation and Manufacturing of PLGA-PTX Nanofibrous Membranes
2.3. Characterization of PLGA-PTX Membranes
2.3.1. Scanning Electron Microscope (SEM) Observation and Fourier Transform Infrared Spectroscopy (FTIR) Assay
2.3.2. Water Contact Angle
2.4. In Vitro Drug Release Study
2.5. Cell Lines and Cell Culture
2.6. In Vivo Study
2.6.1. Orthotopic Xenograft Mouse Model
- Group 1 (DMSO-Ctrl): DMSO solution was administered by weekly intraperitoneal (IP) injection of 100 μL of solvent (10 μL DMSO and 90 μL PBS) for four consecutive weeks.
- Group 2 (DMSO-PTX): Mixed DMSO and PTX solution was administered by weekly IP injection of PTX for 4 consecutive weeks with an equivalent dose of 10 mg/kg (approximately 0.3 mg per mouse) and an injection volume of 100 μL (10 μL of PTX with a concentration of 30 mg/mL and 90 μL of PBS). The total injection dose of PTX was 1.2 mg per mouse (approximately 40 mg/kg).
- Group 3 (PLGA-Ctrl): Blank PLGA nanofibrous membrane was used as the control; it was subcutaneously implanted through a small abdominal incision, which was later closed with 4-0 Vicryl sutures.
- Group 4 (PLGA-PTX): PTX-loaded PLGA nanofibrous membranes with an equivalent PTX dose of 40 mg/kg (approximately 1.2 mg per mouse) were subcutaneously implanted using the same method.
2.6.2. Evaluation of Antitumor Activity
2.6.3. Evaluation of Survival Rate
2.6.4. BLI
2.6.5. Pharmacokinetic Study of PTX
2.6.6. Sample Preparation
2.7. IHC Staining
2.8. Preparation of Total Cell Extracts and Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Preparation and Characterization of PLGA-PTX Nanofibrous Membranes
3.1.1. FTIR
3.1.2. Water Contact Angles of Nanofibers
3.2. In Vitro Drug Release of PTX
3.2.1. Electrospun PLGA Nanofibers Enhanced the Tumor Growth Inhibitory Effects of PTX in TNBC
3.2.2. PTX-Loaded PLGA Nanofibrous Membranes Could Effectively Inhibit TNBC Metastasis and Tumor Progression
3.2.3. Direct Site-Specific Treatment of Breast Cancer by PTX-Loaded PLGA Nanofibrous Membranes Suppresses Angiogenesis
3.2.4. PLGA-PTX Transplantation Contributed to Prolonged Overall Survival Rates
3.2.5. In Vivo Pharmacokinetics of PTX
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmadeka, R.; Harmon, B.E.; Singh, M. Triple-negative breast carcinoma: Current and emerging concepts. Am. J. Clin. Pathol. 2014, 141, 462–477. [Google Scholar] [CrossRef] [Green Version]
- Zaharia, M.; Gomez, H. Triple negative breast cancer: A difficult disease to diagnose and treat. Rev. Peru Med. Exp. Salud Publica 2013, 30, 649–656. [Google Scholar]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, V.; Dieci, M.V.; Conte, P. Relapsed triple-negative breast cancer: Challenges and treatment strategies. Drugs 2013, 73, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Krajnak, S.; Schnatz, C.; Almstedt, K.; Brenner, W.; Haertner, F.; Heimes, A.S.; Lebrecht, A.; Makris, G.M.; Schwab, R.; Hasenburg, A.; et al. Low-dose metronomic chemotherapy as an efficient treatment option in metastatic breast cancer-results of an exploratory case-control study. Breast Cancer Res. Treat. 2020, 182, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.E.; Pinotti, G.; Montagna, E.; Amoroso, D.; Berardi, R.; Butera, A.; Cagossi, K.; Cavanna, L.; Ciccarese, M.; Cinieri, S.; et al. Metronomic chemotherapy for advanced breast cancer patients in the real world practice: Final results of the VICTOR-6 study. Breast 2019, 48, 7–16. [Google Scholar] [CrossRef]
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Gallego Melcon, S.; Merks, J.H.; Kelsey, A.; Martelli, H.; Minard-Colin, V.; Orbach, D.; Glosli, H.; et al. Vinorelbine and continuous low-dose cyclophosphamide as maintenance chemotherapy in patients with high-risk rhabdomyosarcoma (RMS 2005): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019, 20, 1566–1575. [Google Scholar] [CrossRef]
- Intermesoli, T.; Shamash, J.; Rohatiner, A.; Wilson, A.; Lister, A.; Montoto, S. Low dose continuous chemotherapy (LD56): An active treatment with low toxicity for patients with recurrent/refractory lymphoma not eligible for intensive salvage therapy. Br. J. Haematol. 2009, 147, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Andre, N.; Tsai, K.; Carre, M.; Pasquier, E. Metronomic chemotherapy: Direct Targeting of Cancer Cells after all? Trends Cancer 2017, 3, 319–325. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Munzone, E.; Bocci, G.; Afonso, N.; Gomez, P.; Langkjer, S.; Petru, E.; Pivot, X.; Sanchez Rovira, P.; Wysocki, P.; et al. Pan-European expert meeting on the use of metronomic chemotherapy in advanced breast cancer patients: The PENELOPE Project. Adv. Ther. 2019, 36, 381–406. [Google Scholar] [CrossRef]
- Borges, T.C.; Gomes, T.L.N.; Pimentel, G.D. Sarcopenia as a predictor of nutritional status and comorbidities in hospitalized patients with cancer: A cross-sectional study. Nutrition 2020, 73, 110703. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Raza, Z.A.; Nazir, A. Current applications of electrospun polymeric nanofibers in cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.X.; Li, Z.; Yang, Y.; Guo, G.; Luo, F.; Chen, Z.Q.; Yang, Y.; Qian, Z.Y.; Shi, S. Preparation and therapeutic application of docetaxel-loaded poly(d,l-lactide) nanofibers in preventing breast cancer recurrence. Drug Deliv. 2016, 23, 2677–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Bi, X.; Li, H.; Huang, G. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv. 2011, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Liu, D.X.; Zheng, Y.H.; Yue, Y.; Huang, Y.B.; Jing, X.B. Inhibitory effects of paclitaxel-loaded Pla nanofibers against mice cervical cancers. Acta Polym Sin. 2012, 1029–1034. [Google Scholar] [CrossRef]
- Liu, R.; Wolinsky, J.B.; Walpole, J.; Southard, E.; Chirieac, L.R.; Grinstaff, M.W.; Colson, Y.L. Prevention of local tumor recurrence following surgery using low-dose chemotherapeutic polymer films. Ann. Surg. Oncol. 2010, 17, 1203–1213. [Google Scholar] [CrossRef]
- Ma, G.P.; Liu, Y.; Peng, C.; Fang, D.W.; He, B.J.; Nie, J. Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohyd. Polym. 2011, 86, 505–512. [Google Scholar] [CrossRef]
- Xie, J.W.; Wang, C.H. Electrospun micro- and nanofibers for sustained delivery of paclitaxel to treat C6 glioma in vitro. Pharm. Res. 2006, 23, 1817–1826. [Google Scholar] [CrossRef]
- Liu, R.; Wolinsky, J.B.; Catalano, P.J.; Chirieac, L.R.; Wagner, A.J.; Grinstaff, M.W.; Colson, Y.L.; Raut, C.P. Paclitaxel-eluting polymer film reduces locoregional recurrence and improves survival in a recurrent sarcoma model: A novel investigational therapy. Ann. Surg. Oncol. 2012, 19, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.Q.; Liu, C.S.; Li, F.Q.; Ouyang, C.F.; Gao, Q.; Zheng, K.S. Nanoparticles vs. nanofibers: A comparison of two drug delivery systems on assessing drug release performance in vitro. Des. Monomers Polym. 2015, 18, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wei, J.; Yang, C.; Zhao, H.; Li, D.; Yin, Z.; Yang, Z. The inhibition of tumor growth and metastasis by self-assembled nanofibers of taxol. Biomaterials 2012, 33, 5848–5853. [Google Scholar] [CrossRef]
- Xu, H.; Lu, X.; Li, J.; Ding, D.; Wang, H.; Li, X.; Xie, W. Superior antitumor effect of extremely high drug loading self-assembled paclitaxel nanofibers. Int. J. Pharm. 2017, 526, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Smolarczyk, R.; Musial-Kulik, M.; Cichon, T.; Karpeta-Jarzabek, P.; Wlodarczyk, J.; Stojko, M.; Janeczek, H.; Kordyka, A.; Kaczmarczyk, B.; et al. Electrospun paclitaxel delivery system based on PGCL/PLGA in local therapy combined with brachytherapy. Int. J. Pharm. 2021, 602, 120596. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.S.; Chow, P.K.; Wang, C.H. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 2010, 31, 5199–5207. [Google Scholar] [CrossRef]
- Hu, X.L.; Liu, S.; Zhou, G.Y.; Huang, Y.B.; Xie, Z.G.; Jing, X.B. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Richheimer, S.L.; Tinnermeier, D.M.; Timmons, D.W. High-performance liquid chromatographic assay of taxol. Anal. Chem. 1992, 64, 2323–2326. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, B.; Hou, Y.; Yang, J.; Yi, L. Development of a novel morphological paclitaxel-loaded PLGA microspheres for effective cancer therapy: In vitro and in vivo evaluations. Drug Deliv. 2018, 25, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhasz, A.; Ungor, D.; Berta, K.; Seres, L.; Csapo, E. Spreadsheet-based nonlinear analysis of in vitro release properties of a model drug from colloidal carriers. J. Mol. Liq. 2021, 328. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Ginter, P.S.; Karagiannis, G.S.; Entenberg, D.; Lin, Y.; Condeelis, J.; Jones, J.G.; Oktay, M.H. Tumor microenvironment of metastasis (TMEM) doorways are restricted to the blood vessel endothelium in both primary breast cancers and their lymph node metastases. Cancers 2019, 11, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibue, T.; Weinberg, R.A. Metastatic colonization: Settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Semin. Cancer Biol. 2011, 21, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Liebmann, J.E.; Cook, J.A.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J.B. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, N.; Carre, M.; Pasquier, E. Metronomics: Towards personalized chemotherapy? Nat. Rev. Clin. Oncol 2014, 11, 413–431. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Puig, P.E.; Ladoire, S.; Roux, S.; Martin, F.; Solary, E.; Le Cesne, A.; Zitvogel, L.; Chauffert, B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007, 56, 641–648. [Google Scholar] [CrossRef]
- Chan, T.S.; Hsu, C.C.; Pai, V.C.; Liao, W.Y.; Huang, S.S.; Tan, K.T.; Yen, C.J.; Hsu, S.C.; Chen, W.Y.; Shan, Y.S.; et al. Metronomic chemotherapy prevents therapy-induced stromal activation and induction of tumor-initiating cells. J. Exp. Med. 2016, 213, 2967–2988. [Google Scholar] [CrossRef]
- Raymond, E.; Hanauske, A.; Faivre, S.; Izbicka, E.; Clark, G.; Rowinsky, E.K.; Von Hoff, D.D. Effects of prolonged versus short-term exposure paclitaxel (Taxol) on human tumor colony-forming units. Anticancer Drugs 1997, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Masuda, N.; Higaki, K.; Takano, T.; Matsunami, N.; Morimoto, T.; Ohtani, S.; Mizutani, M.; Miyamoto, T.; Kuroi, K.; Ohno, S.; et al. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer. Cancer Chemother. Pharmacol. 2014, 74, 229–238. [Google Scholar] [CrossRef]
- Tao, W.Y.; Liang, X.S.; Liu, Y.; Wang, C.Y.; Pang, D. Decrease of let-7f in low-dose metronomic Paclitaxel chemotherapy contributed to upregulation of thrombospondin-1 in breast cancer. Int. J. Biol. Sci. 2015, 11, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longatto Filho, A.; Lopes, J.M.; Schmitt, F.C. Angiogenesis and breast cancer. J. Oncol. 2010, 2010, 576384. [Google Scholar] [CrossRef]
- Lau, D.H.; Xue, L.; Young, L.J.; Burke, P.A.; Cheung, A.T. Paclitaxel (Taxol): An inhibitor of angiogenesis in a highly vascularized transgenic breast cancer. Cancer Biother. Radiopharm. 1999, 14, 31–36. [Google Scholar] [CrossRef]
- Tomasin, R.; Martin, A.; Cominetti, M.R. Metastasis and cachexia: Alongside in clinics, but not so in animal models. J. Cachexia Sarcopenia Muscle 2019, 10, 1183–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loberg, R.D.; Bradley, D.A.; Tomlins, S.A.; Chinnaiyan, A.M.; Pienta, K.J. The lethal phenotype of cancer: The molecular basis of death due to malignancy. CA Cancer J. Clin. 2007, 57, 225–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffler, T.M.; Freund, W.; Lipke, J.; Hausamen, T.U. Schedule- and dose-intensified paclitaxel as weekly 1-hour infusion in pretreated solid tumors: Results of a phase I/II trial. Semin. Oncol. 1996, 23, 32–34. [Google Scholar] [PubMed]
- Baird, R.D.; Tan, D.S.P.; Kaye, S.B. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat. Rev. Clin. Oncol. 2010, 7, 575–582. [Google Scholar] [CrossRef]
- Alves, R.C.; Fernandes, R.P.; Eloy, J.O.; Salgado, H.R.N.; Chorilli, M. Characteristics, properties and analytical methods of Paclitaxel: A review. Crit. Rev. Anal. Chem. 2018, 48, 110–118. [Google Scholar] [CrossRef]
- Weiss, R.B.; Donehower, R.C.; Wiernik, P.H.; Ohnuma, T.; Gralla, R.J.; Trump, D.L.; Baker, J.R., Jr.; Van Echo, D.A.; Von Hoff, D.D.; Leyland-Jones, B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef]
- Mohamed, F.; Marchettini, P.; Stuart, O.A.; Sugarbaker, P.H. Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemother. Pharmacol. 2003, 52, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Short, A.; Cole, S.L.; Gross, A.C.; Winter, J.; Eubank, T.D.; Lannutti, J.J. Preferential, enhanced breast cancer cell migration on biomimetic electrospun nanofiber ‘cell highways’. BMC Cancer 2014, 14, 825. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Boda, S.K.; Batra, S.K.; Li, X.; Xie, J. Emerging roles of electrospun nanofibers in cancer research. Adv. Healthc. Mater. 2018, 7, e1701024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, F.; Hu, C.; Zhou, Y.; Gao, H.; Hu, J. The progress and perspective of nanoparticle-enabled tumor metastasis treatment. Acta Pharm. Sin. B 2020, 10, 2037–2053. [Google Scholar] [CrossRef] [PubMed]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Asare, S.; Hylton, N.; Van’t Veer, L.; Perlmutter, J.; Wallace, A.M.; Chien, A.J.; Forero-Torres, A.; et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning nanofibers for therapeutics delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-Y.; Hsieh, C.-H.; Huang, Y.-T.; Chu, S.-Y.; Chen, C.-M.; Lee, W.-J.; Liu, S.-J. Enhanced Paclitaxel Efficacy to Suppress Triple-Negative Breast Cancer Progression Using Metronomic Chemotherapy with a Controlled Release System of Electrospun Poly-d-l-Lactide-Co-Glycolide (PLGA) Nanofibers. Cancers 2021, 13, 3350. https://doi.org/10.3390/cancers13133350
Hsu M-Y, Hsieh C-H, Huang Y-T, Chu S-Y, Chen C-M, Lee W-J, Liu S-J. Enhanced Paclitaxel Efficacy to Suppress Triple-Negative Breast Cancer Progression Using Metronomic Chemotherapy with a Controlled Release System of Electrospun Poly-d-l-Lactide-Co-Glycolide (PLGA) Nanofibers. Cancers. 2021; 13(13):3350. https://doi.org/10.3390/cancers13133350
Chicago/Turabian StyleHsu, Ming-Yi, Cheng-Hsien Hsieh, Yu-Ting Huang, Sung-Yu Chu, Chien-Ming Chen, Wei-Jiunn Lee, and Shih-Jung Liu. 2021. "Enhanced Paclitaxel Efficacy to Suppress Triple-Negative Breast Cancer Progression Using Metronomic Chemotherapy with a Controlled Release System of Electrospun Poly-d-l-Lactide-Co-Glycolide (PLGA) Nanofibers" Cancers 13, no. 13: 3350. https://doi.org/10.3390/cancers13133350