Activity-Induced Enhancement of Superdiffusive Transport in Bacterial Turbulence
Abstract
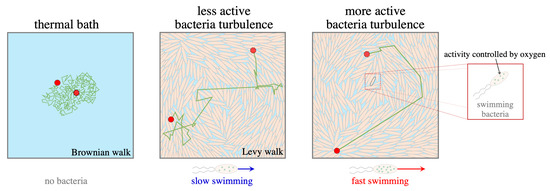
:1. Introduction
2. Materials and Methods
2.1. Bacteria Culture
2.2. Microfluidic Channel and Imaging Method
2.3. Image Processing and Data Analysis
3. Results
3.1. Activity-Induced Enhancement of Superdiffusion
3.2. Superdiffusion in Dilute and Crowed Bacterial Suspensions
3.3. Wall Effect on Superdiffusion
4. Discussion
4.1. Oxygen-Depletion Induced Activity Variation
4.2. Superdiffusion as the Cross-Over Regime
4.3. Activity Effect
4.4. Concentration Effect
4.5. Wall Effect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Redfield, R. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002, 10, 365–370. [Google Scholar] [CrossRef]
- Bos, J.; Cisneros, L.; Mazel, D. Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure. Sci. Adv. 2021, 7, eabd1033. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, A.; Uitenbroek, H.; Boukany, P. Intracellular transport of electrotransferred DNA cargo is governed by coexisting ergodic and non ergodic anomalous diffusion. BioRxiv 2021, 435513, 1–16. [Google Scholar]
- Huda, S.; Weigelin, B.; Wolf, K.; Tretiakov, K.; Polev, K.; Wilk, G.; Iwasa, M.; Emami, F.; Narojczyk, J.; Banaszak, M.; et al. Lévy-like movement patterns of metastatic cancer cells revealed in microfabricated systems and implicated in vivo. Nat. Commun. 2018, 9, 4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sims, D.; Southall, E.; Humphries, N.; Hays, G.; Bradshaw, C.; Pitchford, J.; James, A.; Ahmed, M.; Brierley, A.; Hindell, M.; et al. Scaling laws of marine predator search behaviour. Nature 2008, 451, 1098–1102. [Google Scholar] [CrossRef]
- Reijers, V.; Siteur, K.; Hoeks, S.; Belzen, J.; Borst, A.; Heusinkveld, J.; Govers, L.; Bouma, T.; Lamers, L.; Koppel, J.; et al. A Lévy expansion strategy optimizes early dune building by beach grasses. Nat. Commun. 2019, 10, 2656. [Google Scholar] [CrossRef] [Green Version]
- Shlesinger, M.; Zaslavsky, G.; Klafter, J. Strange kinetics. Nature 1993, 363, 31–37. [Google Scholar] [CrossRef]
- Solomon, T.; Weeks, E.; Swinney, H. Observation of anomalous diffusion and Lévy flights in a two-dimensional rotating flow. Phys. Rev. Lett. 1993, 71, 3975. [Google Scholar] [CrossRef]
- Lukić, B.; Jeney, S.; Tischer, C.; Kulik, A.; Forró, L.; Florin, E. Direct observation of nondiffusive motion of a Brownian particle. Phys. Rev. Lett. 2005, 95, 160601. [Google Scholar] [CrossRef]
- Franosch, T.; Grimm, M.; Belushkin, M.; Mor, F.; Foffi, G.; Forró, L.; Jeney, S. Resonances arising from hydrodynamic memory in Brownian motion. Nature 2011, 478, 85–88. [Google Scholar] [CrossRef]
- Kheifets, S.; Simha, A.; Melin, K.; Li, T.; Raizen, M. Observation of Brownian motion in liquids at short times: Instantaneous velocity and memory loss. Science 2014, 343, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I. Fractional Hydrodynamic Memory and Superdiffusion in Tilted Washboard Potentials. Phys. Rev. Lett. 2019, 123, 180603. [Google Scholar] [CrossRef] [PubMed]
- Goychuk, I.; Pöschel, T. Hydrodynamic memory can boost enormously driven nonlinear diffusion and transport. Phys. Rev. E 2020, 102, 012139. [Google Scholar] [CrossRef] [PubMed]
- Latora, V.; Rapisarda, A.; Ruffo, S. Superdiffusion and out-of-equilibrium chaotic dynamics with many degrees of freedoms. Phys. Rev. Lett. 1999, 83, 2104. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Libchaber, A. Particle diffusion in a quasi-two-dimensional bacterial bath. Phys. Rev. Lett. 2000, 84, 3017. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Ortiz, J.; Stoltz, C.; Graham, M. Transport and Collective Dynamics in Suspensions of Confined Swimming Particles. Phys. Rev. Lett. 2005, 95, 204501. [Google Scholar] [CrossRef] [Green Version]
- Leptos, K.; Guasto, J.; Gollub, J.; Pesci, A.; Goldstein, R. Dynamics of enhanced tracer diffusion in suspensions of swimming eukaryotic microorganisms. Phys. Rev. Lett. 2009, 103, 198103. [Google Scholar] [CrossRef] [Green Version]
- Kurtuldu, H.; Guasto, J.; Johnson, K.; Gollub, J. Enhancement of biomixing by swimming algal cells in two-dimensional films. Proc. Natl. Acad. Sci. USA 2011, 108, 10391–10395. [Google Scholar] [CrossRef] [Green Version]
- Vicsek, T.; Czirók, A.; Ben-Jacob, E.; Cohen, I.; Shochet, O. Novel type of phase transition in a system of self-driven particles. Phys. Rev. Lett. 1995, 75, 1226. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.; Tu, Y. Long-range order in a two-dimensional dynamical XY model: How birds fly together. Phys. Rev. Lett. 1995, 75, 4326. [Google Scholar] [CrossRef]
- Simha, R.; Ramaswamy, S. Hydrodynamic fluctuations and instabilities in ordered suspensions of self-propelled particles. Phys. Rev. Lett. 2002, 89, 058101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saintillan, D.; Shelley, M. Orientational Order and Instabilities in Suspensions of Self-Locomoting Rods. Phys. Rev. Lett. 2007, 99, 058102. [Google Scholar] [CrossRef] [PubMed]
- Saintillan, D.; Shelley, M. Instabilities and Pattern Formation in Active Particle Suspensions: Kinetic Theory and Continuum Simulations. Phys. Rev. Lett. 2008, 100, 178103. [Google Scholar] [CrossRef] [PubMed]
- Underhill, P.; Hernandez-Ortiz, J.; Graham, M. Diffusion and Spatial Correlations in Suspensions of Swimming Particles. Phys. Rev. Lett. 2008, 100, 248101. [Google Scholar] [CrossRef] [Green Version]
- Mino, G.; Mallouk, T.; Darnige, T.; Hoyos, M.; Dauchet, J.; Dunstan, J.; Soto, R.; Wang, Y.; Rousselet, A.; Clement, E. Enhanced diffusion due to active swimmers at a solid surface. Phys. Rev. Lett. 2011, 106, 048102. [Google Scholar] [CrossRef] [Green Version]
- Bechinger, C.; Di Leonardo, R.; Löwen, H.; Reichhardt, C.; Volpe, G.; Volpe, G. Active particles in complex and crowded environments. Rev. Mod. Phys. 2016, 88, 045006. [Google Scholar] [CrossRef]
- Libchaber, A. From biology to physics and back: The problem of Brownian movement. Annu. Rev. Condens. Matter Phys. 2019, 10, 275–293. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, P.; Yang, M.; Hou, M. Experimental investigation of active noise on a rotor in an active granular bath. Soft Matter 2022, 18, 2541–2548. [Google Scholar] [CrossRef]
- Granek, O.; Kafri, Y.; Tailleur, J. The anomalous transport of tracers in active baths. arXiv 2021, arXiv:2108.11970. [Google Scholar]
- Wensink, H.; Dunkel, J.; Heidenreich, S.; Drescher, K.; Goldstein, R.; Löwen, H.; Yeomans, J. Meso-scale turbulence in living fluids. Proc. Natl. Acad. Sci. USA 2012, 109, 14308–14313. [Google Scholar] [CrossRef] [Green Version]
- Wiol, H.; Woodhouse, F.; Dunkel, J.; Kessler, J.; Goldstein, R. Confinement stabilizes a bacterial suspension into a spiral vortex. Phys. Rev. Lett. 2013, 110, 268102. [Google Scholar]
- Wiol, H.; Lushi, E.; Goldstein, R. Directed collective motion of bacteria under channel confinement. New J. Phys. 2016, 18, 075002. [Google Scholar]
- Wu, K.; Hishamunda, J.; Chen, D.; DeCamp, S.; Chang, Y.; Fernández-Nieves, A.; Fraden, S.; Dogic, Z. Transition from turbulent to coherent flows in confined three-dimensional active fluids. Science 2017, 355, eaal1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhu, H.; Zeng, Y.; Du, G.; Ning, L.; Wang, D.; Chen, K.; Lu, Y.; Zheng, N.; Ye, F.; et al. Oscillating collective motion of active rotors in confinement. Proc. Natl. Acad. Sci. USA 2020, 117, 11901–11907. [Google Scholar] [CrossRef]
- Dor, Y.; Ro, S.; Kafri, Y.; Kardar, M.; Tailleur, J. Disordered boundaries destroy bulk phase separation in scalar active matter. Phys. Rev. E 2022, 105, 044603. [Google Scholar]
- Sumpter, D.; Szorkovszky, A.; Kotrschal, A.; Kolm, N.; Herbert-Read, J. Using activity and sociability to characterize collective motion. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170015. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, T.; Chen, D.; DeCamp, S.; Heymann, M.; Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 2012, 491, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Thampi, S.; Golestanian, R.; Yeomans, J. Velocity correlations in an active nematic. Phys. Rev. Lett. 2013, 111, 118101. [Google Scholar] [CrossRef] [Green Version]
- Dunkel, J.; Heidenreich, S.; Drescher, K.; Wensink, H.; Bär, M.; Goldstein, R. Fluid dynamics of bacterial turbulence. Phys. Rev. Lett. 2013, 110, 228102. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, A.; Aranson, I. Physical properties of collective motion in suspensions of bacteria. Phys. Rev. Lett. 2012, 109, 248109. [Google Scholar] [CrossRef]
- Tuval, I.; Cisneros, L.; Dombrowski, C.; Wolgemuth, C.; Kessler, J.; Goldstein, R. Bacterial swimming and oxygen transport near contact lines. Proc. Natl. Acad. Sci. USA 2005, 102, 2277–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezhilan, B.; Pahlavan, A.; Saintillan, D. Chaotic dynamics and oxygen transport in thin films of aerotactic bacteria. Phys. Fluids 2012, 24, 091701. [Google Scholar] [CrossRef]
- Mukherjee, S.; Singh, R.; James, M.; Ray, S. Anomalous diffusion and Lévy walks distinguish active from inertial turbulence. Phys. Rev. Lett. 2021, 127, 118001. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, C.; Li, M.; Novosel, J.; Arlt, J.; Marenduzzo, D. Colloids in a bacterial bath: Simulations and experiments. Soft Matter 2011, 7, 5228–5238. [Google Scholar] [CrossRef] [Green Version]
- Miño, G.; Dunstan, J.; Rousselet, A.; Clément, E.; Soto, R. Induced diffusion of tracers in a bacterial suspension: Theory and experiments. J. Fluid Mech. 2013, 729, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Jepson, A.; Martinez, V.; Schwarz-Linek, J.; Morozov, A.; Poon, W. Enhanced diffusion of nonswimmers in a three-dimensional bath of motile bacteria. Phys. Rev. E 2013, 88, 041002. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Liu, Y.; Luo, H.; Jing, G. Activity-Induced Enhancement of Superdiffusive Transport in Bacterial Turbulence. Micromachines 2022, 13, 746. https://doi.org/10.3390/mi13050746
Xie C, Liu Y, Luo H, Jing G. Activity-Induced Enhancement of Superdiffusive Transport in Bacterial Turbulence. Micromachines. 2022; 13(5):746. https://doi.org/10.3390/mi13050746
Chicago/Turabian StyleXie, Chenliang, Yanan Liu, Hao Luo, and Guangyin Jing. 2022. "Activity-Induced Enhancement of Superdiffusive Transport in Bacterial Turbulence" Micromachines 13, no. 5: 746. https://doi.org/10.3390/mi13050746