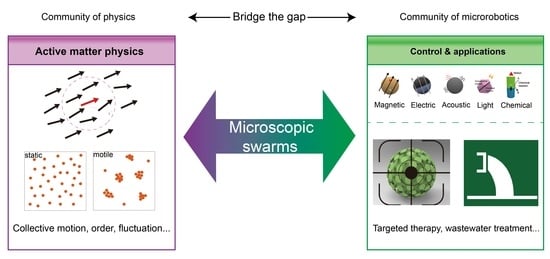
Microscopic Swarms: From Active Matter Physics to Biomedical and Environmental Applications
Abstract
:1. Introduction
2. The Perspective of Fundamental Physics
2.1. Long-Range Order
2.2. Giant Number Fluctuation

2.3. Motility-Induced Phase Separation

2.4. Relationship between Information and Order

3. The Perspective of Application
3.1. Control and Manipulation
3.2. Biomedical Application
3.3. Environmental Application
4. Summary and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballerini, M.; Cabibbo, N.; Candelier, R.; Cavagna, A.; Cisbani, E.; Giardina, I.; Lecomte, V.; Orlandi, A.; Parisi, G.; Procaccini, A.; et al. Interaction Ruling Animal Collective Behavior Depends on Topological Rather than Metric Distance: Evidence from a Field Study. Proc. Natl. Acad. Sci. USA 2008, 105, 1232–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, N.C.; Ratilal, P.; Symonds, D.T.; Jagannathan, S.; Lee, S.; Nero, R.W. Fish Population and Behavior Revealed by Instantaneous Continental Shelf-Scale Imaging. Science 2006, 311, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, N.; Sugawara, K.; Mizuguchi, T.; Hayakawa, Y.; Sano, M. Collective Motion in a System of Motile Elements. Phys. Rev. Lett. 1996, 76, 3870–3873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gueron, S.; Levin, S.A.; Rubenstein, D.I. The Dynamics of Herds: From Individuals to Aggregations. J. Theor. Biol. 1996, 182, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Couzin, I.D.; Krause, J. Self-Organization and Collective Behavior in Vertebrates. In Advances in the Study of Behavior; Academic Press: Cambridge, MA, USA, 2003; Volume 32, pp. 1–75. [Google Scholar]
- Bonabeau, E.; Dorigo, M.; Theraulaz, G. Inspiration for Optimization from Social Insect Behaviour. Nature 2000, 406, 39–42. [Google Scholar] [CrossRef]
- Buhl, J.; Sumpter, D.J.T.; Couzin, I.D.; Hale, J.J.; Despland, E.; Miller, E.R.; Simpson, S.J. From Disorder to Order in Marching Locusts. Science 2006, 312, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, J.A. Thinking about Bacterial Populations as Multicellular Organisms. Annu. Rev. Microbiol. 1998, 52, 81–104. [Google Scholar] [CrossRef] [Green Version]
- Harada, Y.; Noguchi, A.; Kishino, A.; Yanagida, T. Sliding Movement of Single Actin Filaments on One-Headed Myosin Filaments. Nature 1987, 326, 805–808. [Google Scholar] [CrossRef]
- Badoual, M.; Jülicher, F.; Prost, J. Bidirectional Cooperative Motion of Molecular Motors. Proc. Natl. Acad. Sci. USA 2002, 99, 6696–6701. [Google Scholar] [CrossRef] [Green Version]
- Kagan, D.; Balasubramanian, S.; Wang, J. Chemically Triggered Swarming of Gold Microparticles. Angew. Chem. Int. Ed. 2011, 50, 503–506. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B.; Du, X.; Wang, Q.; Zhang, L. Ultra-Extensible Ribbon-like Magnetic Microswarm. Nat. Commun. 2018, 9, 3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesides, G.M.; Grzybowski, B. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cates, M.E.; Tailleur, J. Motility-Induced Phase Separation. Annu. Rev. Condens. Matter Phys. 2015, 6, 219–244. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.; Tu, Y.; Ramaswamy, S. Hydrodynamics and Phases of Flocks. Ann. Phys. 2005, 318, 170–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Luijten, E.; Grzybowski, B.A.; Granick, S. Active Colloids with Collective Mobility Status and Research Opportunities. Chem. Soc. Rev. 2017, 46, 5551–5569. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in Micro-/Nanorobotics: Materials Development, Actuation, Localization, and System Integration for Biomedical Applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, D.; Liang, S.; Dai, Y.; Bai, X.; Song, B.; Zhang, D.; Chen, H.; Feng, L. Recent Advances in Field-Controlled Micro–Nano Manipulations and Micro–Nano Robots. Adv. Intell. Syst. 2021, 2100116. [Google Scholar] [CrossRef]
- Ji, F.; Li, T.; Yu, S.; Wu, Z.; Zhang, L. Propulsion Gait Analysis and Fluidic Trapping of Swinging Flexible Nanomotors. ACS Nano 2021, 15, 5118–5128. [Google Scholar] [CrossRef]
- Yu, H.; Tang, W.; Mu, G.; Wang, H.; Chang, X.; Dong, H.; Qi, L.; Zhang, G.; Li, T. Micro-/Nanorobots Propelled by Oscillating Magnetic Fields. Micromachines 2018, 9, 540. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Chen, C.; Li, J.; Lu, X.; Liang, Y.; Zhou, D.; Wang, H.; Zhang, G.; Li, T.; Wang, J.; et al. Motile Micropump Based on Synthetic Micromotors for Dynamic Micropatterning. ACS Appl. Mater. Interfaces 2019, 11, 28507–28514. [Google Scholar] [CrossRef]
- Li, T.; Zhang, A.; Shao, G.; Wei, M.; Guo, B.; Zhang, G.; Li, L.; Wang, W. Janus Microdimer Surface Walkers Propelled by Oscillating Magnetic Fields. Adv. Funct. Mater. 2018, 28, 1706066. [Google Scholar] [CrossRef]
- Yang, L.; Yu, J.; Yang, S.; Wang, B.; Nelson, B.J.; Zhang, L. A Survey on Swarm Microrobotics. IEEE Trans. Robot. 2021, 1–21. [Google Scholar] [CrossRef]
- Liu, C.; Xu, T.; Xu, L.-P.; Zhang, X. Controllable Swarming and Assembly of Micro/Nanomachines. Micromachines 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. Nanomachines: Fundamentals and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33120-8. [Google Scholar]
- Sitti, M. Mobile Microrobotics; Intelligent Robotics and Autonomous Agents series; MIT Press: Cambridge, MA, USA, 2017; ISBN 978-0-262-03643-6. [Google Scholar]
- Li, J.; Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto–Acoustic Hybrid Nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gao, Y.; Yang, J.; Li, Y.C.; Shao, G.; Zhang, G.; Li, T.; Li, L. Light-Ultrasound Driven Collective “Firework” Behavior of Nanomotors. Adv. Sci. 2018, 5, 1800122. [Google Scholar] [CrossRef]
- Battle, C.; Broedersz, C.P.; Fakhri, N.; Geyer, V.F.; Howard, J.; Schmidt, C.F.; MacKintosh, F.C. Broken Detailed Balance at Mesoscopic Scales in Active Biological Systems. Science 2016, 352, 604–607. [Google Scholar] [CrossRef] [Green Version]
- Mermin, N.D.; Wagner, H. Absence of Ferromagnetism or Antiferromagnetism in One- or Two-Dimensional Isotropic Heisenberg Models. Phys. Rev. Lett. 1966, 17, 1133–1136. [Google Scholar] [CrossRef]
- Vicsek, T.; Czirók, A.; Ben-Jacob, E.; Cohen, I.; Shochet, O. Novel Type of Phase Transition in a System of Self-Driven Particles. Phys. Rev. Lett. 1995, 75, 1226–1229. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.W. Flocks, Herds and Schools: A Distributed Behavioral Model. ACM SIGGRAPH Comput. Graph. 1987, 21, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.; Tu, Y. Long-Range Order in a Two-Dimensional Dynamical XY Model: How Birds Fly Together. Phys. Rev. Lett. 1995, 75, 4326–4329. [Google Scholar] [CrossRef]
- Tu, Y.; Toner, J.; Ulm, M. Sound Waves and the Absence of Galilean Invariance in Flocks. Phys. Rev. Lett. 1998, 80, 4819–4822. [Google Scholar] [CrossRef] [Green Version]
- Toner, J.; Tu, Y. Flocks, Herds, and Schools: A Quantitative Theory of Flocking. Phys. Rev. E 1998, 58, 4828–4858. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, D.; Nagai, K.H.; Chaté, H.; Sano, M. Long-Range Nematic Order and Anomalous Fluctuations in Suspensions of Swimming Filamentous Bacteria. Phys. Rev. E 2017, 95, 020601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruler, H.; Dewald, U.; Eberhardt, M. Nematic Liquid Crystals Formed by Living Amoeboid Cells. Eur. Phys. J. B 1999, 11, 187–192. [Google Scholar] [CrossRef]
- Kemkemer, R.; Kling, D.; Kaufmann, D.; Gruler, H. Elastic Properties of Nematoid Arrangements Formed by Amoeboid Cells. Eur. Phys. J. E 2000, 1, 215–225. [Google Scholar] [CrossRef]
- Deseigne, J.; Dauchot, O.; Chaté, H. Collective Motion of Vibrated Polar Disks. Phys. Rev. Lett. 2010, 105, 098001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bricard, A.; Caussin, J.-B.; Desreumaux, N.; Dauchot, O.; Bartolo, D. Emergence of Macroscopic Directed Motion in Populations of Motile Colloids. Nature 2013, 503, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Shani, I.; Beatus, T.; Bar-Ziv, R.H.; Tlusty, T. Long-Range Orientational Order in Two-Dimensional Microfluidic Dipoles. Nat. Phys. 2014, 10, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Deseigne, J.; Léonard, S.; Dauchot, O.; Chaté, H. Vibrated Polar Disks: Spontaneous Motion, Binary Collisions, and Collective Dynamics. Soft Matter 2012, 8, 5629–5639. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Diamant, H.; Lin, B.; Rice, S.A. Anomalous Hydrodynamic Interaction in a Quasi-Two-Dimensional Suspension. Phys. Rev. Lett. 2004, 92, 258301. [Google Scholar] [CrossRef] [Green Version]
- Mahault, B.; Chaté, H. Long-Range Nematic Order in Two-Dimensional Active Matter. Phys. Rev. Lett. 2021, 127, 048003. [Google Scholar] [CrossRef] [PubMed]
- Gompper, G.; Winkler, R.G.; Speck, T.; Solon, A.; Nardini, C.; Peruani, F.; Löwen, H.; Golestanian, R.; Kaupp, U.B.; Alvarez, L.; et al. The 2020 Motile Active Matter Roadmap. J. Phys. Condens. Matter 2020, 32, 193001. [Google Scholar] [CrossRef] [PubMed]
- Chaté, H.; Ginelli, F.; Montagne, R. Simple Model for Active Nematics: Quasi-Long-Range Order and Giant Fluctuations. Phys. Rev. Lett. 2006, 96, 180602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toner, J. Giant Number Fluctuations in Dry Active Polar Fluids: A Shocking Analogy with Lightning Rods. J. Chem. Phys. 2019, 150, 154120. [Google Scholar] [CrossRef] [Green Version]
- Narayan, V.; Ramaswamy, S.; Menon, N. Long-Lived Giant Number Fluctuations in a Swarming Granular Nematic. Science 2007, 317, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Aranson, I.S.; Snezhko, A.; Olafsen, J.S.; Urbach, J.S. Comment on “Long-Lived Giant Number Fluctuations in a Swarming Granular Nematic”. Science 2008, 320, 612. [Google Scholar] [CrossRef] [Green Version]
- Kudrolli, A.; Lumay, G.; Volfson, D.; Tsimring, L.S. Swarming and Swirling in Self-Propelled Polar Granular Rods. Phys. Rev. Lett. 2008, 100, 058001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.P.; Be’er, A.; Florin, E.-L.; Swinney, H.L. Collective Motion and Density Fluctuations in Bacterial Colonies. Proc. Natl. Acad. Sci. USA 2010, 107, 13626–13630. [Google Scholar] [CrossRef] [Green Version]
- Peruani, F.; Starruß, J.; Jakovljevic, V.; Søgaard-Andersen, L.; Deutsch, A.; Bär, M. Collective Motion and Nonequilibrium Cluster Formation in Colonies of Gliding Bacteria. Phys. Rev. Lett. 2012, 108, 098102. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, W.; Ma, X.; Cheng, X. Density Fluctuations and Energy Spectra of 3D Bacterial Suspensions. Soft Matter 2021, 17, 10806–10817. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kageyama, R.; Sano, M. Topological Defects Control Collective Dynamics in Neural Progenitor Cell Cultures. Nature 2017, 545, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, F.; Malinverno, C.; Corallino, S.; Ginelli, F.; Scita, G.; Cerbino, R. Giant Fluctuations and Structural Effects in a Flocking Epithelium. J. Phys. Appl. Phys. 2017, 50, 384003. [Google Scholar] [CrossRef] [Green Version]
- Karani, H.; Pradillo, G.E.; Vlahovska, P.M. Tuning the Random Walk of Active Colloids: From Individual Run-and-Tumble to Dynamic Clustering. Phys. Rev. Lett. 2019, 123, 208002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacci, J.; Sacanna, S.; Steinberg, A.P.; Pine, D.J.; Chaikin, P.M. Living Crystals of Light-Activated Colloidal Surfers. Science 2013, 339, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Simha, R.A.; Toner, J. Active Nematics on a Substrate: Giant Number Fluctuations and Long-Time Tails. Europhys. Lett. 2003, 62, 196. [Google Scholar] [CrossRef] [Green Version]
- Chaté, H.; Ginelli, F.; Grégoire, G.; Peruani, F.; Raynaud, F. Modeling Collective Motion: Variations on the Vicsek Model. Eur. Phys. J. B 2008, 64, 451–456. [Google Scholar] [CrossRef]
- Chaté, H.; Ginelli, F.; Grégoire, G.; Raynaud, F. Collective Motion of Self-Propelled Particles Interacting without Cohesion. Phys. Rev. E 2008, 77, 046113. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.C.; Joanny, J.F.; Ramaswamy, S.; Liverpool, T.B.; Prost, J.; Rao, M.; Simha, R.A. Hydrodynamics of Soft Active Matter. Rev. Mod. Phys. 2013, 85, 1143–1189. [Google Scholar] [CrossRef] [Green Version]
- Berg, H.C.; Brown, D.A. Chemotaxis in Escherichia Coli Analysed by Three-Dimensional Tracking. Nature 1972, 239, 500–504. [Google Scholar] [CrossRef]
- Romanczuk, P.; Bär, M.; Ebeling, W.; Lindner, B.; Schimansky-Geier, L. Active Brownian Particles. Eur. Phys. J. Spec. Top. 2012, 202, 1–162. [Google Scholar] [CrossRef] [Green Version]
- Moran, J.L.; Posner, J.D. Phoretic Self-Propulsion. Annu. Rev. Fluid Mech. 2017, 49, 511–540. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum Sensing in Bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnitzer, M.J. Theory of Continuum Random Walks and Application to Chemotaxis. Phys. Rev. E 1993, 48, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Tailleur, J.; Cates, M.E. Statistical Mechanics of Interacting Run-and-Tumble Bacteria. Phys. Rev. Lett. 2008, 100, 218103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cates, M.E.; Tailleur, J. When Are Active Brownian Particles and Run-and-Tumble Particles Equivalent? Consequences for Motility-Induced Phase Separation. EPL Europhys. Lett. 2013, 101, 20010. [Google Scholar] [CrossRef] [Green Version]
- Wittkowski, R.; Tiribocchi, A.; Stenhammar, J.; Allen, R.J.; Marenduzzo, D.; Cates, M.E. Scalar Φ4 Field Theory for Active-Particle Phase Separation. Nat. Commun. 2014, 5, 4351. [Google Scholar] [CrossRef] [Green Version]
- Fily, Y.; Marchetti, M.C. Athermal Phase Separation of Self-Propelled Particles with No Alignment. Phys. Rev. Lett. 2012, 108, 235702. [Google Scholar] [CrossRef] [Green Version]
- Buttinoni, I.; Bialké, J.; Kümmel, F.; Löwen, H.; Bechinger, C.; Speck, T. Dynamical Clustering and Phase Separation in Suspensions of Self-Propelled Colloidal Particles. Phys. Rev. Lett. 2013, 110, 238301. [Google Scholar] [CrossRef] [Green Version]
- Bäuerle, T.; Fischer, A.; Speck, T.; Bechinger, C. Self-Organization of Active Particles by Quorum Sensing Rules. Nat. Commun. 2018, 9, 3232. [Google Scholar] [CrossRef]
- van der Linden, M.N.; Alexander, L.C.; Aarts, D.G.A.L.; Dauchot, O. Interrupted Motility Induced Phase Separation in Aligning Active Colloids. Phys. Rev. Lett. 2019, 123, 098001. [Google Scholar] [CrossRef] [Green Version]
- Theurkauff, I.; Cottin-Bizonne, C.; Palacci, J.; Ybert, C.; Bocquet, L. Dynamic Clustering in Active Colloidal Suspensions with Chemical Signaling. Phys. Rev. Lett. 2012, 108, 268303. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Patch, A.; Bahar, F.; Yllanes, D.; Welch, R.D.; Marchetti, M.C.; Thutupalli, S.; Shaevitz, J.W. Self-Driven Phase Transitions Drive Myxococcus Xanthus Fruiting Body Formation. Phys. Rev. Lett. 2019, 122, 248102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Han, M.; Zhang, J.; Xu, C.; Luijten, E.; Granick, S. Reconfiguring Active Particles by Electrostatic Imbalance. Nat. Mater. 2016, 15, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Pei, A.; Dong, R.; Wang, J. Catalytic Iridium-Based Janus Micromotors Powered by Ultralow Levels of Chemical Fuels. J. Am. Chem. Soc. 2014, 136, 2276–2279. [Google Scholar] [CrossRef] [Green Version]
- Ibele, M.; Mallouk, T.E.; Sen, A. Schooling Behavior of Light-Powered Autonomous Micromotors in Water. Angew. Chem. Int. Ed. 2009, 48, 3308–3312. [Google Scholar] [CrossRef]
- Duan, W.; Liu, R.; Sen, A. Transition between Collective Behaviors of Micromotors in Response to Different Stimuli. J. Am. Chem. Soc. 2013, 135, 1280–1283. [Google Scholar] [CrossRef]
- Devreotes, P. Dictyostelium Discoideum: A Model System for Cell-Cell Interactions in Development. Science 1989, 245, 1054–1058. [Google Scholar] [CrossRef]
- Eichinger, L.; Pachebat, J.A.; Glöckner, G.; Rajandream, M.-A.; Sucgang, R.; Berriman, M.; Song, J.; Olsen, R.; Szafranski, K.; Xu, Q.; et al. The Genome of the Social Amoeba Dictyostelium Discoideum. Nature 2005, 435, 43–57. [Google Scholar] [CrossRef] [Green Version]
- Cates, M.E.; Marenduzzo, D.; Pagonabarraga, I.; Tailleur, J. Arrested Phase Separation in Reproducing Bacteria Creates a Generic Route to Pattern Formation. Proc. Natl. Acad. Sci. USA 2010, 107, 11715–11720. [Google Scholar] [CrossRef] [Green Version]
- Kardar, M. Statistical Physics of Fields; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; ISBN 978-0-521-87341-3. [Google Scholar]
- Martiniani, S.; Chaikin, P.M.; Levine, D. Quantifying Hidden Order out of Equilibrium. Phys. Rev. X 2019, 9, 011031. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Gardi, G.; Malgaretti, P.; Kishore, V.; Koens, L.; Son, D.; Gilbert, H.; Wu, Z.; Harwani, P.; Lauga, E.; et al. Order and Information in the Patterns of Spinning Magnetic Micro-Disks at the Air-Water Interface. Sci. Adv. 2022, 8, eabk0685. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Giltinan, J.; Zakharchenko, S.; Sitti, M. Dynamic and Programmable Self-Assembly of Micro-Rafts at the Air-Water Interface. Sci. Adv. 2017, 3, e1602522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Kishore, V.; Koens, L.; Lauga, E.; Sitti, M. Collectives of Spinning Mobile Microrobots for Navigation and Object Manipulation at the Air-Water Interface. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; 2018; pp. 1–9. [Google Scholar]
- Koens, L.; Wang, W.; Sitti, M.; Lauga, E. The near and Far of a Pair of Magnetic Capillary Disks. Soft Matter 2019, 15, 1497–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, L. Motion Control in Magnetic Microrobotics: From Individual and Multiple Robots to Swarms. Annu. Rev. Control Robot. Auton. Syst. 2021, 4, 509–534. [Google Scholar] [CrossRef]
- Xie, H.; Sun, M.; Fan, X.; Lin, Z.; Chen, W.; Wang, L.; Dong, L.; He, Q. Reconfigurable Magnetic Microrobot Swarm: Multimode Transformation, Locomotion, and Manipulation. Sci. Robot. 2019. [Google Scholar] [CrossRef]
- Zhou, Z.; Hou, Z.; Pei, Y. Reconfigurable Particle Swarm Robotics Powered by Acoustic Vibration Tweezer. Soft Robot. 2021, 8, 735–743. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Fitzner, K.; Paczesny, J.; Granick, S. From Dynamic Self-Assembly to Networked Chemical Systems. Chem. Soc. Rev. 2017, 46, 5647–5678. [Google Scholar] [CrossRef]
- Soo Kim, P.S.; Becker, A.; Ou, Y.; Julius, A.A.; Kim, M.J. Swarm Control of Cell-Based Microrobots Using a Single Global Magnetic Field. In Proceedings of the 2013 10th International Conference on Ubiquitous Robots and Ambient Intelligence (URAI), Jeju, Korea, 31 October–2 November 2013; 2013; pp. 21–26. [Google Scholar]
- Dong, X.; Sitti, M. Controlling Two-Dimensional Collective Formation and Cooperative Behavior of Magnetic Microrobot Swarms. Int. J. Robot. Res. 2020, 39, 617–638. [Google Scholar] [CrossRef]
- Radiation: Electromagnetic Fields. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-electromagnetic-fields (accessed on 12 January 2022).
- Magdaleno-Adame, S.; Olivares-Galvan, J.C.; Campero-Littlewood, E.; Escarela-Perez, R.; Blanco-Brisset, E.; No, C. Coil Systems to Generate Uniform Magnetic Field Volumes. 7. Available online: https://cds.comsol.com/paper/download/101163/olivares_paper.pdf?__gda__=1644744428_c12b01129ec24513285249ab427ebeeb&fileExt=.pdf (accessed on 20 January 2022).
- Albrecht, D.R.; Underhill, G.H.; Wassermann, T.B.; Sah, R.L.; Bhatia, S.N. Probing the Role of Multicellular Organization in Three-Dimensional Microenvironments. Nat. Methods 2006, 3, 369–375. [Google Scholar] [CrossRef]
- Lee, H.-S.; Go, G.; Choi, E.; Kang, B.; Park, J.-O.; Kim, C.-S. Medical Microrobot—Wireless Manipulation of a Drug Delivery Carrier through an External Ultrasonic Actuation: Preliminary Results. Int. J. Control Autom. Syst. 2020, 18, 175–185. [Google Scholar] [CrossRef]
- Sitti, M.; Wiersma, D.S. Pros and Cons: Magnetic versus Optical Microrobots. Adv. Mater. 2020, 32, 1906766. [Google Scholar] [CrossRef] [PubMed]
- Paxton, W.F.; Sen, A.; Mallouk, T.E. Motility of Catalytic Nanoparticles through Self-Generated Forces. Chem.–Eur. J. 2005, 11, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Chude-Okonkwo, U.A.K. Diffusion-Controlled Enzyme-Catalyzed Molecular Communication System for Targeted Drug Delivery. In Proceedings of the 2014 IEEE Global Communications Conference, Austin, TX, USA, 8–12 December 2014; 2014; pp. 2826–2831. [Google Scholar]
- Chan, V.; Harry Asada, H.; Bashir, R. Utilization and Control of Bioactuators across Multiple Length Scales. Lab. Chip 2014, 14, 653–670. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, L.; Yu, J.; Vong, C.-I.; Yan Chiu, P.W.; Zhang, L. Magnetic Navigation of a Rotating Colloidal Swarm Using Ultrasound Images. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; 2018; pp. 5380–5385. [Google Scholar]
- Snezhko, A.; Belkin, M.; Aranson, I.S.; Kwok, W.-K. Self-Assembled Magnetic Surface Swimmers. Phys. Rev. Lett. 2009, 102, 118103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snezhko, A.; Aranson, I.S. Magnetic Manipulation of Self-Assembled Colloidal Asters. Nat. Mater. 2011, 10, 698–703. [Google Scholar] [CrossRef]
- Martel, S.; Taherkhani, S.; Tabrizian, M.; Mohammadi, M.; de Lanauze, D.; Felfoul, O. Computer 3D Controlled Bacterial Transports and Aggregations of Microbial Adhered Nano-Components. J. Micro-Bio Robot. 2014, 9, 23–28. [Google Scholar] [CrossRef]
- Park, B.-W.; Zhuang, J.; Yasa, O.; Sitti, M. Multifunctional Bacteria-Driven Microswimmers for Targeted Active Drug Delivery. ACS Nano 2017, 11, 8910–8923. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Xu, J.; Zhou, J.; Liu, J.; Ye, M.; Zhang, L.; Qiao, B.; Wang, Z.; Ran, H.; et al. Low-Intensity Focused Ultrasound-Responsive Phase-Transitional Nanoparticles for Thrombolysis without Vascular Damage: A Synergistic Nonpharmaceutical Strategy. ACS Nano 2019, 13, 3387–3403. [Google Scholar] [CrossRef]
- Manamanchaiyaporn, L.; Xu, T.; Wu, X. An Optimal Design of an Electromagnetic Actuation System towards a Large Homogeneous Magnetic Field and Accessible Workspace for Magnetic Manipulation. Energies 2020, 13, 911. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Chan, K.F.; Yu, J.; Wang, Q.; Yang, L.; Chiu, P.W.Y.; Zhang, L. Reconfigurable Swarms of Ferromagnetic Colloids for Enhanced Local Hyperthermia. Adv. Funct. Mater. 2018, 28, 1705701. [Google Scholar] [CrossRef]
- Servant, A.; Qiu, F.; Mazza, M.; Kostarelos, K.; Nelson, B.J. Controlled In Vivo Swimming of a Swarm of Bacteria-Like Microrobotic Flagella. Adv. Mater. 2015, 27, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, L.; Yuan, K.; Ji, F.; Gao, J.; Zhang, Z.; Du, X.; Tian, Y.; Wang, Q.; Zhang, L. Magnetic Microswarm Composed of Porous Nanocatalysts for Targeted Elimination of Biofilm Occlusion. ACS Nano 2021, 15, 5056–5067. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, W.; Fan, X.; Tian, C.; Sun, L.; Xie, H. Cooperative Recyclable Magnetic Microsubmarines for Oil and Microplastics Removal from Water. Appl. Mater. Today 2020, 20, 100682. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, K.; Ji, F.; Zhang, L. Enhanced Removal of Toxic Heavy Metals Using Swarming Biohybrid Adsorbents. Adv. Funct. Mater. 2018, 28, 1806340. [Google Scholar] [CrossRef]
- Wu, Z.; Troll, J.; Jeong, H.-H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018. [Google Scholar] [CrossRef] [Green Version]
- Devlin, P.M. Brachytherapy: Applications and Techniques; Springer Publishing Company: New York, NY, USA, 2015; ISBN 978-1-61705-261-3. [Google Scholar]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for Minimally Invasive Medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [Green Version]
- Andrä, W.; Nowak, H. Magnetism in Medicine: A Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-3-527-61018-1. [Google Scholar]
- Manamanchaiyaporn, L.; Tang, X.; Zheng, Y.; Yan, X. Molecular Transport of a Magnetic Nanoparticle Swarm Towards Thrombolytic Therapy. IEEE Robot. Autom. Lett. 2021, 6, 5605–5612. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.-X.J.; et al. Multifunctional Biohybrid Magnetite Microrobots for Imaging-Guided Therapy. Sci. Robot. 2017. [Google Scholar] [CrossRef] [Green Version]
- Hwang, G.; Paula, A.J.; Hunter, E.E.; Liu, Y.; Babeer, A.; Karabucak, B.; Stebe, K.; Kumar, V.; Steager, E.; Koo, H. Catalytic Antimicrobial Robots for Biofilm Eradication. Sci. Robot. 2019. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Mou, F.; Pan, D.; Chen, C.; Gao, Y.; Xu, L.; Guan, J. Magnetically Modulated Pot-Like MnFe2O4 Micromotors: Nanoparticle Assembly Fabrication and Their Capability for Direct Oil Removal. Adv. Funct. Mater. 2015, 25, 6173–6181. [Google Scholar] [CrossRef]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-Propelled Micromotors for Cleaning Polluted Water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Villa, K.; Vilela, D.; Sánchez, S. Platinum-Free Cobalt Ferrite Based Micromotors for Antibiotic Removal. Appl. Mater. Today 2017, 9, 605–611. [Google Scholar] [CrossRef]
- Delezuk, J.A.; Ramírez-Herrera, D.E.; de Ávila, B.E.-F.; Wang, J. Chitosan-Based Water-Propelled Micromotors with Strong Antibacterial Activity. Nanoscale 2017, 9, 2195–2200. [Google Scholar] [CrossRef]
- Villa, K.; Děkanovský, L.; Plutnar, J.; Kosina, J.; Pumera, M. Swarming of Perovskite-Like Bi2WO6 Microrobots Destroy Textile Fibers under Visible Light. Adv. Funct. Mater. 2020, 30, 2007073. [Google Scholar] [CrossRef]
- Zhou, H.; Mayorga-Martinez, C.C.; Pumera, M. Microplastic Removal and Degradation by Mussel-Inspired Adhesive Magnetic/Enzymatic Microrobots. Small Methods 2021, 5, 2100230. [Google Scholar] [CrossRef]
- Moo, J.G.S.; Pumera, M. Chemical Energy Powered Nano/Micro/Macromotors and the Environment. Chem.–Eur. J. 2015, 21, 58–72. [Google Scholar] [CrossRef]
- Zhao, G.; Khezri, B.; Sanchez, S.; Schmidt, O.G.; D. Webster, R.; Pumera, M. Corrosion of Self-Propelled Catalytic Microengines. Chem. Commun. 2013, 49, 9125–9127. [Google Scholar] [CrossRef]
- Nagai, K.H. Collective Motion of Rod-Shaped Self-Propelled Particles through Collision. Biophys. Physicobiol. 2018, 15, 51–57. [Google Scholar] [CrossRef] [Green Version]




| Type of System | Model (Characteristic Size) | Reference | |
|---|---|---|---|
| Millimeter-scale particles | Granular rods (d = 0.8/l = 4.6 mm) | 1 | [48] |
| Spherical particles (d = 1.0 mm) | 1 | [49] | |
| Polar granular rods (d = 4.8/l = 9.5 mm) | 0.66 | [50] | |
| Polar disks (d = 4 mm) | 0.8 | [39,42] | |
| Bacteria | Cylindrical Bacillus subtilis (l = 5.0/d = 1.0 µm) | [51] | |
| Rod-shaped Myxococcus xanthus (l = 6.3/d = 0.7 µm) | 0.85 | [52] | |
| Filamentous Escherichia coli (l = 20/d = 0.8 µm) | 0.63 | [36] | |
| Escherichia coli in quasi-3D ) | 0.83 | [53] | |
| Cells | Neural progenitor cell (l = 100/d = 10 µm) | 0.75 | [54] |
| Flocking epithelium (d = 30 ) | 0.8 | [55] | |
| Colloidal particles | ) | 0.85 | [56] |
| Photoactivated colloid (d = 1.5 µm) | 0.9 | [57] |
| Type of System | Model (Characteristic Size) | Primary Swarming Mechanism | References |
|---|---|---|---|
| Artificial system (Near-zero attractive interaction) | Light-activated carbon-coated Janus particles (d = 4 µm) in near-critical water-lutidine mixture | MIPS | [71,72] |
| Artificial system (significant attractive interaction) | Light-activated polymer (TPM) sphere (d = 1.5 µm) | Osmotically-driven motion and collision | [57] |
| ) in deionized water controlled by a.c. electric fields | Induced-charge electrophoresis | [73,76] | |
| Ir/SiO2 Janus particles (d = 1.2 µm) with low level of hydrazine | Diffusioosmotic Diffusiophoresis | [77] | |
| Au particles (d = 1 µm) in H2O2 solution spiked with hydrazine | Diffusiophoresis Diffusioosmosis Electrophoresis Electroosmosis | [11] | |
| Spherical gold colloids half covered with platinum in H2O2 solution (d = 1 µm) | [74] | ||
| UV-activated AgCl particle (d = 1 µm) in deionized water | [78] | ||
| Ag3PO4 microparticle (d = 2 µm) schooling controlled by addition or removal of NH3 | [79] | ||
| Biological world | Bacteria Myxococcus xanthus (l = 5 µm) | Quorum sensing/ Chemotaxis | [75] |
| Bacteria Dictyostelium discoideum (l = 20 µm) | [80,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Yu, H.; Zhang, X.; Malgaretti, P.; Kishore, V.; Wang, W. Microscopic Swarms: From Active Matter Physics to Biomedical and Environmental Applications. Micromachines 2022, 13, 295. https://doi.org/10.3390/mi13020295
Fu Y, Yu H, Zhang X, Malgaretti P, Kishore V, Wang W. Microscopic Swarms: From Active Matter Physics to Biomedical and Environmental Applications. Micromachines. 2022; 13(2):295. https://doi.org/10.3390/mi13020295
Chicago/Turabian StyleFu, Yulei, Hengao Yu, Xinli Zhang, Paolo Malgaretti, Vimal Kishore, and Wendong Wang. 2022. "Microscopic Swarms: From Active Matter Physics to Biomedical and Environmental Applications" Micromachines 13, no. 2: 295. https://doi.org/10.3390/mi13020295