Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor
Abstract
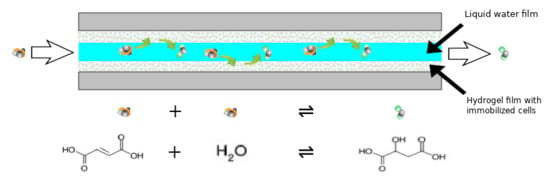
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganism Cultivation and Preparation
2.3. Cell Immobilization in Sodium Alginate-Polyvinyl Alcohol (SA-PVA) Copolymeric Hydrogel
2.4. Characterization of SA-PVA Copolymeric Hydrogel
2.5. Microreactor Assembly
2.6. Determination of the Immobilization Effectiveness Factor
2.7. Biotransformation in a Microreactor
2.8. The Effect of Temperature on Biotransformation in a Microreactor
2.9. Determination of Biotransformation System Stability
2.10. High-Performance Liquid Chromatography (HPLC) Analysis of Fumaric Acid Concentration
3. Results
3.1. Characterization of SA-PVA Copolymer Hydrogel
3.2. Determination of the Immobilization Effectiveness Factor
3.3. Biotransformation within a Microbioreactor
3.4. Determination of System Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wohlgemuth, R.; Plazl, I.; Žnidaršič-Plazl, P.; Gernaey, K.V.; Woodley, J.M. Microscale technology and biocatalytic processes: Opportunities and challenges for synthesis. Trends Biotechnol. 2015, 33, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Pohar, A.; Plazl, I. Process intensification through microreactor application. Chem. Biochem. Eng. Q. 2009, 23, 537–544. [Google Scholar]
- Žnidaršič-Plazl, P. The promises and the challenges of biotransformations in microflow. Biotechnol. J. 2019, 14, 1800580. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Valikhani, D.; Nidetzky, B. Demystifying the Flow: Biocatalytic reaction intensification in microstructured enzyme reactors. Biotechnol. J. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Stojkovič, G.; Žnidaršič-Plazl, P. Continuous synthesis of l-malic acid using whole-cell microreactor. Process Biochem. 2012, 47, 1102–1107. [Google Scholar] [CrossRef]
- Žnidaršič-Plazl, P. Biotransformations in Microflow Systems: Bridging the gap between academia and industry. J. Flow Chem. 2017, 7, 111–117. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today 2016, 259, 66–80. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Miložič, N.; Stojkovič, G.; Vogel, A.; Bouwes, D.; Žnidaršič-Plazl, P. Development of microreactors with surface-immobilized biocatalysts for continuous transamination. New Biotechnol. 2018, 47, 18–24. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, C.T.; Xu, Y.; Wang, F.Q.; Tsabing, D.K.; Wu, F.A.; Wang, J. Moving and unsinkable graphene sheets immobilized enzyme for microfluidic biocatalysis. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajić, M.; Plazl, I.; Stloukal, R.; Žnidaršič-Plazl, P. Development of a miniaturized packed bed reactor with ω-transaminase immobilized in LentiKats®. Process Biochem. 2017, 52, 63–72. [Google Scholar]
- Dai, Y.; Niu, J.; Yin, L.; Liu, J.; Jiang, G. Electrospun nanofiber membranes as supports for enzyme immobilization and its application. Prog. Chem. 2010, 22, 1808–1818. [Google Scholar]
- Liu, L.S.; Kost, J.; Yan, F.; Spiro, R.C. Hydrogels from biopolymer hybrid for biomedical, food, and functional food applications. Polymers 2012, 4, 997–1011. [Google Scholar] [CrossRef]
- Fernandez-Arrojo, L.; Rodriguez-Colinas, B.; Gutierrez-Alonso, P.; Fernandez-Lobato, M.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Dried alginate-entrapped enzymes (DALGEEs) and their application to the production of fructooligosaccharides. Process Biochem. 2013, 48, 677–682. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.E.; Lassalle, V.; Ferreira, M.L. FTIR-ATR characterization of free Rhizomucor meihei lipase (RML), Lipozyme RM IM and chitosan-immobilized RML. J. Mol. Catal. B Enzym. 2011, 72, 220–228. [Google Scholar] [CrossRef]
- Chi, M.C.; Lyu, R.C.; Lin, L.L.; Huang, H.B. Characterization of Bacillus kaustophilus leucine aminopeptidase immobilized in Ca-alginate/k-carrageenan beads. Biochem. Eng. J. 2008, 39, 376–382. [Google Scholar] [CrossRef]
- Kim, M.H.; An, S.; Won, K.; Kim, H.J.; Lee, S.H. Entrapment of enzymes into cellulose-biopolymer composite hydrogel beads using biocompatible ionic liquid. J. Mol. Catal. B Enzym. 2012, 75, 68–72. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Gois, P.M.P.; Rosa, M.E.; Martins, S.; Fernandes, P.C.B.; Ribeiro, M.H.L. Boronic acids as efficient cross linkers for PVA: Synthesis and application of tunable hollow microspheres in biocatalysis. Tetrahedron 2016, 72, 7293–7305. [Google Scholar] [CrossRef]
- Azmi, N.; Azmi, W. Entrapment of purified novel dextransucrase obtained from newly isolated Acetobacter tropicalis and its comparative study of kinetic parameters with free enzyme. Biocatal. Biotransform. 2019, 37, 349–360. [Google Scholar]
- Liao, H.; Liu, Y.; Wang, Q.; Duan, W. Structure and properties of porous poly (vinyl alcohol) hydrogel beads prepared through a physical–chemical crosslinking method. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J. Environ. Radioact. 2016, 162–163, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Engler, C.R.; Wild, J.R. Biodegradation of coumaphos, chlorferon, and diethylthiophosphate using bacteria immobilized in Ca-alginate gel beads. Bioresour. Technol. 2009, 100, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, I.; Parshad, R.; Qazi, G.N.; Gupta, V.K. Immobilization of lipase by entrapment in Ca-alginate beads. J. Bioact. Compat. Polym. 2008, 23, 552–562. [Google Scholar] [CrossRef]
- Nunes, M.A.P.; Vila-Real, H.; Fernandes, P.C.B.; Ribeiro, M.H.L. Immobilization of naringinase in PVA-alginate matrix using an innovative technique. Appl. Biochem. Biotechnol. 2010, 160, 2129–2147. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Panke, S.; Schmid, A. The production of fine chemicals by biotransformations. Chem. Biotechnol. 2002, 13, 548–556. [Google Scholar] [CrossRef]
- Chen, R.R. Permeability issues in whole-cell bioprocesses and cellular membrane engineering. Appl. Microbiol. Biotechnol. 2007, 74, 730–738. [Google Scholar] [CrossRef]
- Vrsalović Presečki, A.; Zelić, B.; Vasić-Rački, Đ. Comparison of the L-malic acid production by isolated fumarase and fumarase in permeabilized baker’s yeast cells. Enzym. Microb. Technol. 2007, 41, 605–612. [Google Scholar] [CrossRef]
- Vasić-Rački, Đ. The novel technologies for the use of biocatalyst and biotransformations. Prog. Chem. 2005, 2, 1–8. [Google Scholar]
- Hronská, H.; Tokošová, S.; Pilniková, A.; Krištofíková, Ľ.; Rosenberg, M. Bioconversion of fumaric acid to L-malic acid by the bacteria of the genus nocardia. Appl. Biochem. Biotechnol. 2015, 175, 266–273. [Google Scholar] [CrossRef]
- Vrsalović Presečki, A.; Zelić, B.; Vasić-Rački, Đ. Modelling of continuous L-malic acid production by porcine heart fumarase and fumarase in yeast cells. Chem. Biochem. Eng. Q. 2009, 23, 519–525. [Google Scholar]
- Stojkovič, G.; Plazl, I.; Žnidaršič-Plazl, P. L-Malic acid production within a microreactor with surface immobilised fumarase. Microfluid. Nanofluid. 2011, 10, 627–635. [Google Scholar]
- Franck, A. Understanding Rheology of Structured Fluids. 2004. Available online: http://www.tainstruments.com/pdf/literature/AAN016_V1_U_StructFluids.pdf (accessed on 19 September 2019).
- Kazenwadel, F.; Wagner, H.; Rapp, B.E.; Franzreb, M. Optimization of enzyme immobilization on magnetic microparticles using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) as a crosslinking agent. Anal. Methods 2015, 7, 10291–10298. [Google Scholar] [CrossRef] [Green Version]
- Zakhartsev, M.; Reuss, M. Cell size and morphological properties of yeast Saccharomyces cerevisiae in relation to growth temperature. FEMS Yeast Res. 2018, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]







| Hydrogel Name | Polyvinyl Alcohol (PVA) Concentration [% (w/v)] | Sodium Alginate (SA) Concentration [% (w/v)] | Crosslinker |
|---|---|---|---|
| 4/2-BA | 4 | 2 | Boric acid |
| 8/2-BA | 8 | 2 | Boric acid |
| 12/2-BA | 12 | 2 | Boric acid |
| 4/2-PBA | 4 | 2 | Phenylboronic acid |
| 8/2-PBA | 8 | 2 | Phenylboronic acid |
| 12/2-PBA | 12 | 2 | Phenylboronic acid |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegatti, T.; Žnidaršič-Plazl, P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines 2019, 10, 867. https://doi.org/10.3390/mi10120867
Menegatti T, Žnidaršič-Plazl P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines. 2019; 10(12):867. https://doi.org/10.3390/mi10120867
Chicago/Turabian StyleMenegatti, Tadej, and Polona Žnidaršič-Plazl. 2019. "Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor" Micromachines 10, no. 12: 867. https://doi.org/10.3390/mi10120867