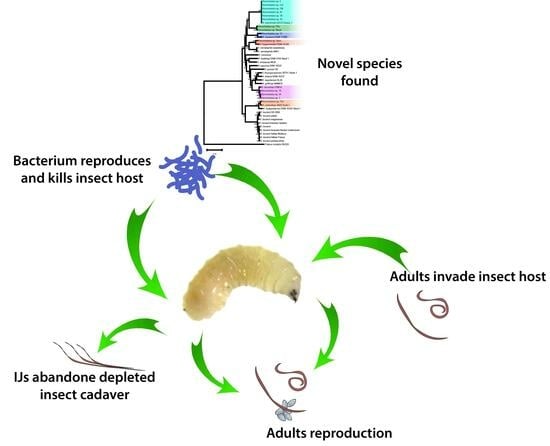
Genome Sequence Analysis of Native Xenorhabdus Strains Isolated from Entomopathogenic Nematodes in Argentina
Abstract
:1. Introduction
2. Results
2.1. Isolation of Bacteria and Preliminary Identification
2.2. Genome Assembly and Phylogenetic Analysis
2.3. Gene Prediction and Annotation of Virulence Factors
2.4. Prediction of Biosynthetic Gene Clusters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Collection of Soil Samples and Isolation of Bacteria
5.2. DNA Isolation and Genome Sequencing
5.3. Genome Assembly and Annotation
5.4. Genome–Genome Comparisons and Phylogenetic Analysis
5.5. Insecticidal Gene Annotation
5.6. Prediction of Biosynthetic Gene Clusters
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jurat-Fuentes, J.L.; Jackson, T.A. Bacterial Entomopathogens. In Insect Pathology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Ruiu, L. Insect Pathogenic Bacteria in Integrated Pest Management. Insects 2015, 6, 352–367. [Google Scholar] [CrossRef]
- Labaude, S.; Griffin, C.T. Transmission Success of Entomopathogenic Nematodes Used in Pest Control. Insects 2018, 9, 72. [Google Scholar] [CrossRef]
- Tobias, N.J.; Wolff, H.; Djahanschiri, B.; Grundmann, F.; Kronenwerth, M.; Shi, Y.M.; Simonyi, S.; Grun, P.; Shapiro-Ilan, D.; Pidot, S.J.; et al. Natural product diversity associated with the nematode symbionts Photorhabdus and Xenorhabdus. Nat. Microbiol. 2017, 2, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.t.; Rowedder, H.; Michaels, B.; Bullock, H.; Jackobeck, R.; Abebe-Akele, F.; Durakovic, U.; Gately, J.; Janicki, E.; Tisa, L.S. Elucidation of the Photorhabdus temperata Genome and Generation of a Transposon Mutant Library To Identify Motility Mutants Altered in Pathogenesis. J. Bacteriol. 2015, 197, 2201–2216. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-LeRoux, C.; Gaudriault, S.; Ramarao, N.; Lereclus, D.; Givaudan, A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr. Opin. Microbiol. 2012, 15, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Inman, F.L., 3rd; Singh, S.; Holmes, L.D. Mass Production of the Beneficial Nematode Heterorhabditis bacteriophora and Its Bacterial Symbiont Photorhabdus luminescens. Indian J. Microbiol. 2012, 52, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Federhen, S. The NCBI Taxonomy database. Nucleic Acids Res. 2012, 40, D136–D143. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Kumar, M.; Kumari, P.; Mahapatro, G.K.; Banerjee, N.; Sarin, N.B. Novel insecticidal chitinase from the insect pathogen Xenorhabdus nematophila. Int. J. Biol. Macromol. 2020, 159, 394–401. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Photorhabdus spp.: An Overview of the Beneficial Aspects of Mutualistic Bacteria of Insecticidal Nematodes. Plants 2021, 10, 1660. [Google Scholar] [CrossRef]
- da Silva, W.J.; Pilz-Junior, H.L.; Heermann, R.; da Silva, O.S. The great potential of entomopathogenic bacteria Xenorhabdus and Photorhabdus for mosquito control: A review. Parasit. Vectors 2020, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Waterfield, N.R. The role of TcdB and TccC subunits in secretion of the Photorhabdus Tcd toxin complex. PLoS Pathog. 2013, 9, e1003644. [Google Scholar] [CrossRef] [PubMed]
- Daborn, P.J.; Waterfield, N.; Silva, C.P.; Au, C.P.; Sharma, S.; Ffrench-Constant, R.H. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. USA 2002, 99, 10742–10747. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, R.H.; Dowling, A.; Waterfield, N.R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 2007, 49, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Schubert, E.; Vetter, I.R.; Prumbaum, D.; Penczek, P.A.; Raunser, S. Membrane insertion of alpha-xenorhabdolysin in near-atomic detail. eLife 2018, 7, e38017. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Cao, A.T.; Dobson, P.; Hines, E.R.; Akhurst, R.J.; East, P.D. Txp40, a ubiquitous insecticidal toxin protein from Xenorhabdus and Photorhabdus bacteria. Appl. Environ. Microbiol. 2006, 72, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, P.; Zhang, J.; Nangong, Z.; Liu, X.; Gao, Y.; Wang, Q. Characteristics and Function of the Chitin Binding Protein from Xenorhabdus nematophila. Protein Pept. Lett. 2019, 26, 414–422. [Google Scholar] [CrossRef]
- Park, Y.; Kyo Jung, J.; Kim, Y. A Mixture of Bacillus thuringiensis subsp. israelensis With Xenorhabdus nematophila-Cultured Broth Enhances Toxicity Against Mosquitoes Aedes albopictus and Culex pipiens pallens (Diptera: Culicidae). J. Econ. Entomol. 2016, 109, 1086–1093. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y. Synergistic Effect of Entomopathogenic Bacteria (Xenorhabdus sp. and Photorhabdus temperata ssp. temperata) on the Pathogenicity of Bacillus thuringiensis ssp. aizawai Against Spodoptera exigua (Lepidoptera: Noctuidae). Environ. Entomol. 2006, 35, 1584–1589. [Google Scholar] [CrossRef]
- NanGong, Z.; Wang, Q.Y.; Song, P.; Hao, J.; Yang, Q.; Wang, L. Synergism between Bacillus thuringiensis and Xenorhabdus nematophila against resistant and susceptible Plutella xylostella (Lepidoptera: Plutellidae). Biocontrol Sci. Technol. 2016, 26, 1411–1419. [Google Scholar] [CrossRef]
- Booysen, E.; Rautenbach, M.; Stander, M.A.; Dicks, L.M.T. Profiling the Production of Antimicrobial Secondary Metabolites by Xenorhabdus khoisanae J194 Under Different Culturing Conditions. Front. Chem. 2021, 9, 626653. [Google Scholar] [CrossRef]
- Isaacson, P.J.; Webster, J.M. Antimicrobial activity of Xenorhabdus sp. RIO (Enterobacteriaceae), symbiont of the entomopathogenic nematode, Steinernema riobrave (Rhabditida: Steinernematidae). J. Invertebr. Pathol. 2002, 79, 146–153. [Google Scholar] [CrossRef]
- Chaston, J.M.; Suen, G.; Tucker, S.L.; Andersen, A.W.; Bhasin, A.; Bode, E.; Bode, H.B.; Brachmann, A.O.; Cowles, C.E.; Cowles, K.N.; et al. The entomopathogenic bacterial endosymbionts Xenorhabdus and Photorhabdus: Convergent lifestyles from divergent genomes. PLoS ONE 2011, 6, e27909. [Google Scholar] [CrossRef] [PubMed]
- Mothupi, B.; Featherston, J.; Gray, V. Draft Whole-Genome Sequence and Annotation of Xenorhabdus griffiniae Strain BMMCB Associated with the South African Entomopathogenic Nematode Steinernema khoisanae Strain BMMCB. Genome Announc. 2015, 3, e00785-15. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, S.; Featherston, J.; Gray, V.M. Draft Whole-Genome Sequence and Annotation of the Entomopathogenic Bacterium Xenorhabdus khoisanae Strain MCB. Genome Announc. 2015, 3, e00872-15. [Google Scholar] [CrossRef] [PubMed]
- Soobramoney, L.A.; Featherston, J.; Gray, V.M. Draft Whole-Genome Sequence of Xenorhabdus sp. Strain GDc328, Isolated from the Indigenous South African Nematode Host Steinernema khoisanae. Genome Announc. 2015, 3, e01239-15. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Stoilova-McPhie, S.; Baxter, L.; Fulop, V.; Henderson, J.; Rodger, A.; Roper, D.I.; Scott, D.J.; Smith, C.J.; Morgan, J.A. Structural characterisation of the insecticidal toxin XptA1, reveals a 1.15 MDa tetramer with a cage-like structure. J. Mol. Biol. 2007, 366, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Hurst, M.R.; Jones, S.A.; Binglin, T.; Harper, L.A.; Jackson, T.A.; Glare, T.R. The main virulence determinant of Yersinia entomophaga MH96 is a broad-host-range toxin complex active against insects. J. Bacteriol. 2011, 193, 1966–1980. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A structure-based nomenclature for Bacillus thuringiensis and other bacteria-derived pesticidal proteins. J. Invertebr. Pathol. 2020, 107438. [Google Scholar] [CrossRef]
- Ullah, I.; Jang, E.K.; Kim, M.S.; Shin, J.H.; Park, G.S.; Khan, A.R.; Hong, S.J.; Jung, B.K.; Choi, J.; Park, Y.; et al. Identification and characterization of the insecticidal toxin “makes caterpillars floppy” in Photorhabdus temperata M1021 using a cosmid library. Toxins 2014, 6, 2024–2040. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Li, Y.; Ding, X.; Yang, Q.; Sun, Y.; Yu, Z.; Xia, L.; Hu, S. XaxAB-like binary toxin from Photorhabdus luminescens exhibits both insecticidal activity and cytotoxicity. FEMS Microbiol. Lett. 2014, 350, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Pascal Andreu, V.; de Los Santos, E.L.C.; Del Carratore, F.; Lee, S.Y.; Medema, M.H.; Weber, T. The antiSMASH database version 2: A comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2019, 47, D625–D630. [Google Scholar] [CrossRef] [PubMed]
- Meesil, W.; Muangpat, P.; Sitthisak, S.; Rattanarojpong, T.; Chantratita, N.; Machado, R.A.R.; Shi, Y.-M.; Bode, H.B.; Vitta, A.; Thanwisai, A. Genome mining reveals novel biosynthetic gene clusters in entomopathogenic bacteria. Sci. Rep. 2023, 13, 20764. [Google Scholar] [CrossRef] [PubMed]
- McQuade, R.; Stock, P. Secretion Systems and Secreted Proteins in Gram-Negative Entomopathogenic Bacteria: Their Roles in Insect Virulence and Beyond. Insects 2018, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Sampson, K.; Zaitseva, J.; Stauffer, M.; Vande Berg, B.; Guo, R.; Tomso, D.; McNulty, B.; Desai, N.; Balasubramanian, D. Discovery of a novel insecticidal protein from Chromobacterium piscinae, with activity against Western Corn Rootworm, Diabrotica virgifera virgifera. J. Invertebr. Pathol. 2017, 142, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bazok, R.; Lemic, D.; Chiarini, F.; Furlan, L. Western Corn Rootworm (Diabrotica virgifera virgifera LeConte) in Europe: Current Status and Sustainable Pest Management. Insects 2021, 12, 195. [Google Scholar] [CrossRef]
- Subramanian, S.; Muthulakshmi, M. Chapter 12—Entomopathogenic Nematodes. In Ecofriendly Pest Management for Food Security; Omkar, O., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 367–410. [Google Scholar]
- Moazami, N. Biological Control. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 772–784. [Google Scholar]
- Blackburn, M.B.; Farrar, R.R.; Novak, N.G.; Lawrence, S.D. Remarkable susceptibility of the diamondback moth (Plutella xylostella) to ingestion of Pir toxins from Photorhabdus luminescens. Entomol. Exp. Appl. 2006, 121, 31–37. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, S.; Ma, X.; Baxter, S.W.; Vasseur, L.; Xiong, L.; Huang, Y.; Yang, G.; You, S.; You, M. Resistance to Bacillus thuringiensis Cry1Ac toxin requires mutations in two Plutella xylostella ATP-binding cassette transporter paralogs. PLoS Pathog. 2020, 16, e1008697. [Google Scholar] [CrossRef]
- Ahantarig, A.; Chantawat, N.; Waterfield, N.R.; Ffrench-Constant, R.; Kittayapong, P. PirAB toxin from Photorhabdus asymbiotica as a larvicide against dengue vectors. Appl. Environ. Microbiol. 2009, 75, 4627–4629. [Google Scholar] [CrossRef] [PubMed]
- Smigielski, A.J.; Akhurst, R.J. Megaplasmids in Xenorhabdus and Photorhabdus spp., bacterial symbionts of Entomopathogenic nematodes (Families Steirnernematidae and Heterorhabditidae). J. Invertebr. Pathol. 1994, 64, 214–220. [Google Scholar] [CrossRef]
- Park, Y.; Kang, S.; Sadekuzzaman, M.; Kim, H.; Jung, J.K.; Kim, Y. Identification and bacterial characteristics of Xenorhabdus hominickii ANU101 from an entomopathogenic nematode, Steinernema monticolum. J. Invertebr. Pathol. 2017, 144, 74–87. [Google Scholar] [CrossRef]
- Dreyer, J.; Malan, A.P.; Dicks, L.M.T. Bacteria of the Genus Xenorhabdus, a Novel Source of Bioactive Compounds. Front. Microbiol. 2018, 9, 3177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Peng, D.; Ruan, L.; Sun, M. Evolution and dynamics of megaplasmids with genome sizes larger than 100 kb in the Bacillus cereus group. BMC Evol. Biol. 2013, 13, 262. [Google Scholar] [CrossRef]
- Ding, X.; Gopalakrishnan, B.; Johnson, L.B.; White, F.F.; Wang, X.; Morgan, T.D.; Kramer, K.J.; Muthukrishnan, S. Insect resistance of transgenic tobacco expressing an insect chitinase gene. Transgenic. Res. 1998, 7, 77–84. [Google Scholar] [CrossRef]
- Chacon-Orozco, J.G.; Bueno, C.J.; Shapiro-Ilan, D.I.; Hazir, S.; Leite, L.G.; Harakava, R. Antifungal activity of Xenorhabdus spp. and Photorhabdus spp. against the soybean pathogenic Sclerotinia sclerotiorum. Sci. Rep. 2020, 10, 20649. [Google Scholar] [CrossRef]
- Gualtieri, M.; Aumelas, A.; Thaler, J.O. Identification of a new antimicrobial lysine-rich cyclolipopeptide family from Xenorhabdus nematophila. J. Antibiot. 2009, 62, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Abebew, D.; Sayedain, F.S.; Bode, E.; Bode, H.B. Uncovering Nematicidal Natural Products from Xenorhabdus Bacteria. J. Agric. Food. Chem. 2022, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Booysen, E.; Dicks, L.M.T. Does the Future of Antibiotics Lie in Secondary Metabolites Produced by Xenorhabdus spp.? A Review. Probiotics Antimicrob. Proteins 2020, 12, 1310–1320. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Bedding, R.A.; Akhurst, R.J. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 1975, 21, 109–110. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Biological Techniques Series; Academic Press: London, UK, 1997; pp. 281–324. [Google Scholar]
- Stevens, G.; Erdogan, H.; Pimentel, E.; Dotson, J.; Stevens, A.; Shapiro-Ilan, D.; Kaplan, F.; Schliekelman, P.; Lewis, E. Group joining behaviours in the entomopathogenic nematode Steinernema glaseri. Biol. Control 2023, 181, 105220. [Google Scholar] [CrossRef]
- Del Valle, E.E.; Balbi, E.I.; Lax, P.; Rondán Dueñas, J.; Doucet, M.E. Ecological aspects of an isolate of Steinernema diaprepesi (Rhabditida: Steinernematidae) from Argentina. Biocontrol Sci. Technol. 2014, 24, 690–704. [Google Scholar] [CrossRef]
- Akhurst, R.J. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J. Gen. Microbiol. 1980, 121, 303–309. [Google Scholar] [CrossRef]
- Del Valle, E.E.; Frizzo, L.S.; Malmierca, M.; Zbrun, M.V.; Lax, P.; Doucet, M.E. Biological control of Alphitobius diaperinus with Steinernema rarum CUL and Heterorhabditis bacteriophora SMC and feasibility of application in rice hull. J. Pest. Sci. 2016, 89, 161–1701. [Google Scholar] [CrossRef]
- Zerbino, D.R. Using the Velvet de novo assembler for short-read sequencing technologies. Curr. Protoc. Bioinform. 2010, 31, 11.5.1–11.5.12. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Stock, S.P. A multigene approach for assessing evolutionary relationships of Xenorhabdus spp. (gamma-Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. J. Invertebr. Pathol. 2010, 104, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Sayers, E.W.; Cavanaugh, M.; Clark, K.; Pruitt, K.D.; Schoch, C.L.; Sherry, S.T.; Karsch-Mizrachi, I. GenBank. Nucleic Acids. Res. 2021, 49, D92–D96. [Google Scholar] [CrossRef]
- Rodriguez, R.L.M.; Konstantinidis, K.T. The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ Prepr. 2016, 4, e1900v1. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Goker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids. Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Palma, L.; Del Valle, E.E.; Frizzo, L.; Berry, C.; Caballero, P. Draft Genome Sequence of Photorhabdus luminescens Strain DSPV002N Isolated from Santa Fe, Argentina. Genome Announc. 2016, 4, e00744-16. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. Methods Mol. Biol. 2017, 1607, 627–641. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2020, 49, 412–419. [Google Scholar] [CrossRef]
- Shi, Y.M.; Hirschmann, M.; Shi, Y.N.; Ahmed, S.; Abebew, D.; Tobias, N.J.; Grun, P.; Crames, J.J.; Poschel, L.; Kuttenlochner, W.; et al. Global analysis of biosynthetic gene clusters reveals conserved and unique natural products in entomopathogenic nematode-symbiotic bacteria. Nat. Chem. 2022, 14, 701–712. [Google Scholar] [CrossRef] [PubMed]



| Strains (This Study) | Reference 16S rRNA | Nematode Host | GenBank Acc. No. | % Pairwise Identity |
|---|---|---|---|---|
| 5 | X. szentirmaii | Steneinerma sp. | FJ515803 | 99 |
| Cul | X. szentirmaii | S. rarum | FJ515803 | 99 |
| ZM | X. szentirmaii | Steneinerma sp. | FJ515803 | 99 |
| M | X. szentirmaii | S. rarum | FJ515803 | 99 |
| 38 | X. szentirmaii | S. rarum | FJ515803 | 99 |
| 42 | X. szentirmaii | S. rarum | FJ515803 | 99 |
| PSL | X. szentirmaii | Steneinerma sp. | FJ515803 | 98 |
| Reich | X. szentirmaii | Steneinerma sp. | FJ515802 | 99 |
| 12 | X. mauleonii | Steneinerma sp. | NR_043645 | 99 |
| Vera | X. szentirmaii | Steneinerma sp. | FJ515802 | 99 |
| 18 | X. doucetiae | S. diaprepesi | FO704550 | 99 |
| DI | X. doucetiae | S. diaprepesi | DQ211702 | 99 |
| 3 | X. doucetiae | Steneinerma sp. | DQ211702 | 99 |
| Flor | X. cabanillasii | Steneinerma sp. | DQ211711 | 99 |
| Strain | Multigene Approach | Species (%ANI) | Species TYGS | Proposed Species |
|---|---|---|---|---|
| Xenorhabdus sp. 5 | X. szentirmaii | X. szentirmaii (99.47) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. Cul | X. szentirmaii | X. szentirmaii (99.65) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. ZM | X. szentirmaii | X. szentirmaii (99.64) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. M | X. szentirmaii | X. szentirmaii (99.64) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. 38 | X. szentirmaii | X. szentirmaii (99.38) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. 42 | X. szentirmaii | X. szentirmaii (99.74) | X. szentirmaii | X. szentirmaii |
| Xenorhabdus sp. PSL * | Unknown | Unknown (≤85.00) a | Unknown | X. littoralis |
| Xenorhabdus sp. Reich * | Unknown | Unknown (≤85.00) a | Unknown | X. littoralis |
| Xenorhabdus sp. 12 | X. mauleonii | Unknown (90.23, ≤85.00) b | Unknown | X. santafensis |
| Xenorhabdus sp. Vera | X. koppenhoeferi | X. koppenhoeferi (96.69) | X. koppenhoeferi | X. koppenhoeferi |
| Xenorhabdus sp. 18 | X. doucetiae | X. doucetiae (97.25) | X. doucetiae | X. doucetiae |
| Xenorhabdus sp. DI | X. doucetiae | X. doucetiae (97.23) | X. doucetiae | X. doucetiae |
| Xenorhabdus sp. 3 | X. doucetiae | X. doucetiae (97.25) | X. doucetiae | X. doucetiae |
| Xenorhabdus sp. Flor | X. cabanillasi | X. cabanillasi (97.46) | X. cabanillasii | X. cabanillasi |
| Genomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | 5 | Cul | ZM | M | 38 | 42 | PSL | Reich | 12 | Vera | 18 | DI | 3 | Flor |
| Size (bp) | 4,704,623 | 4,811,834 | 4,937,636 | 4,895,665 | 4,855,573 | 4,856,095 | 4,270,206 | 4,511,286 | 4,697,413 | 4,394,775 | 4,274,643 | 4,178,992 | 4,194,235 | 4,405,897 |
| Contigs | 439 | 809 | 675 | 594 | 396 | 684 | 629 | 336 | 550 | 701 | 674 | 466 | 513 | 553 |
| CDs | 4271 | 4246 | 4476 | 4485 | 4499 | 4329 | 3873 | 3899 | 4127 | 3861 | 3760 | 3756 | 3748 | 3908 |
| GC% | 43.7 | 43.3 | 43.7 | 43.6 | 43.9 | 43.6 | 43.1 | 43.6 | 43.4 | 43.0 | 45.0 | 45.4 | 45.4 | 42.8 |
| Tc | 8 | 9 | 7 | 5 | 17 | 8 | - | 8 | 1 | 2 | 7 | 6 | 6 | 2 |
| Txp | - | - | - | - | - | - | 1 | - | - | 1 | - | - | - | 1 |
| Mcf | 1 | - | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | - | 1 | 1 |
| Pra/Prb | - | - | - | - | - | - | - | - | - | 1 | 1 | 1 | 1 | 1 |
| App | - | 1 | - | - | - | - | - | - | 1 | - | - | - | - | 1 |
| iProtein a | - | - | - | - | - | - | 1 | 1 | - | 1 | - | - | - | - |
| nProteins b | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Chitinases | 2 | 4 | 4 | 4 | 3 | 4 | - | - | 1 | - | 2 | 2 | 2 | - |
| Species | X. szentirmaii | X. littoralis | X. santafensis | X. koppenhoeferi | X. doucetiae | X. cabanillasi | ||||||||
| Strains | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene Clusters Manually Annotated from Bode’s Lab In-House Database [35] | Function | 5 | Cul | ZM | M | 38 | 42 | PSL | Reich | 12 | Vera | 18 | DI | 3 | Flor |
| Aryl polyene | Antioxidative | ||||||||||||||
| β-lactone | Proteasome inhibitor | ||||||||||||||
| GameXPeptide | Insect immunosuppressive | ||||||||||||||
| Rhabdobranin | Unknown | ||||||||||||||
| Xenorhabdin | Proteasome inhibitor | ||||||||||||||
| PAX lipopeptides | Antifungal | ||||||||||||||
| Lipocitide | Insect immunosuppressive | ||||||||||||||
| Xenobactin | Antimicrobial | ||||||||||||||
| Photoxenobactin | Siderophore/insecticidal | ||||||||||||||
| Xenoamicin | Antiprotozoal | ||||||||||||||
| Phenazine | Antibiotic | 2 | 2 | 2 | |||||||||||
| Szentirazine | Unknown | ||||||||||||||
| Fabclavine | Antimicrobial | ||||||||||||||
| ATRed | Unknown | 2 | 2 | 2 | 2 | 2 | 3 | 2 | 2 | ||||||
| Pyrrolizixenamide | antibiotic | ||||||||||||||
| Xenocoumacin | Antifungal/antibiotic | ||||||||||||||
| Photopyrone | Quorum sensing | ||||||||||||||
| Photobactin | Siderophore | ||||||||||||||
| Safracin | Antibiotic | ||||||||||||||
| Putrebactin/Avaroferrin | Iron uptake | 2 | 2 | ||||||||||||
| Xefoampeptide | Insect immunosuppressive | ||||||||||||||
| Xenotetrapeptide | Insect immunosuppressive | ||||||||||||||
| Species | X. szentirmaii | X. littoralis | Xs | Xk | X. doucetiae | Xc | |||||||||
| Strain | City, Department, Province | Soil Sample |
|---|---|---|
| 5 | Videla, San Justo, Santa Fe | Soybean-cultivated soil |
| Cul | Cululú, Las Colonias, Santa Fe, | Corn-cultivated soil |
| ZM | Esperanza, Las Colonias, Santa Fe, | Corn-cultivated soil |
| M | La Criolla, San Justo, Santa Fe | Soybean-cultivated soil |
| 38 | Sarmiento, Las Colonias, Santa Fe | Corn-cultivated soil |
| 42 | Esperanza, Las Colonias, Santa Fe | Wheat-cultivated soil |
| PSL | Paso de los Libres, Paso de los Libres, Corrientes | Grassland soil |
| Reich | Gualeguay, Gualeguay, Entre Ríos | Soybean-cultivated soil |
| 12 | Marcelino Escalada, San Justo, Santa Fe | Native soil |
| Vera | Vera, Vera, Santa Fe | Native carob forest with cattle. |
| 18 | Colonia Campo del Medio, Garay, Santa Fe | Zucchini-cultivated soil |
| DI | Santa Rosa de Calchines, Garay, Santa Fe | Carrot-cultivated soil |
| 3 | Cabal, La Capital, Santa Fe | Soybean-cultivated soil |
| Flor | Florencia, General Obligado, Santa Fe | Sugar cane-cultivated soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, L.; Frizzo, L.; Kaiser, S.; Berry, C.; Caballero, P.; Bode, H.B.; Del Valle, E.E. Genome Sequence Analysis of Native Xenorhabdus Strains Isolated from Entomopathogenic Nematodes in Argentina. Toxins 2024, 16, 108. https://doi.org/10.3390/toxins16020108
Palma L, Frizzo L, Kaiser S, Berry C, Caballero P, Bode HB, Del Valle EE. Genome Sequence Analysis of Native Xenorhabdus Strains Isolated from Entomopathogenic Nematodes in Argentina. Toxins. 2024; 16(2):108. https://doi.org/10.3390/toxins16020108
Chicago/Turabian StylePalma, Leopoldo, Laureano Frizzo, Sebastian Kaiser, Colin Berry, Primitivo Caballero, Helge B. Bode, and Eleodoro Eduardo Del Valle. 2024. "Genome Sequence Analysis of Native Xenorhabdus Strains Isolated from Entomopathogenic Nematodes in Argentina" Toxins 16, no. 2: 108. https://doi.org/10.3390/toxins16020108