B-Type Fumonisins in Post-Fermented Tea: Occurrence and Consumer Dietary Exposure in Guangxi, China
Abstract
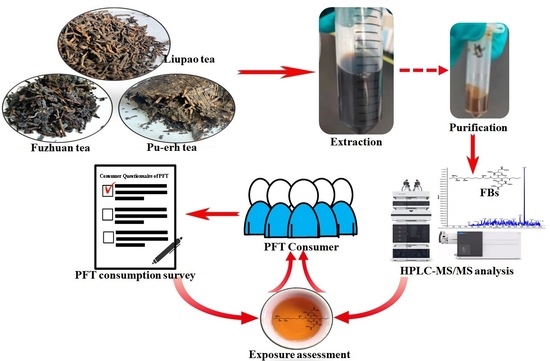
:1. Introduction
2. Results and Discussion
2.1. Method Development and Validation
2.2. Occurrence and Concentration of FBs in PFT
2.3. Demographic Profile and PFT Consumption Patterns in Guangxi
2.4. Exposure Assessment and Risk Characterization
3. Conclusions
4. Materials and Methods
4.1. Sources of PFT Samples, Chemicals, and Reagents
4.2. Sample Pre-Treatment
4.3. Sample Analysis
4.4. PFT Consumption Survey
4.5. Exposure Assessment
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency of Research on Cancer. Mycotoxins and human health. In Improving Public Health through Mycotoxin Control; IARC: Lyon, France, 2012; pp. 87–104. [Google Scholar]
- Li, R.; Tao, B.; Pang, M.; Liu, Y.; Dong, J. Natural occurrence of fumonisins B1 and B2 in maize from three main maize-producing provinces in China. Food Control 2015, 50, 838–842. [Google Scholar] [CrossRef]
- Wang, L.M.; Huang, D.F.; Fang, Y.; Wang, F.; Li, F.L.; Liao, M. Soil fungal communities in tea plantation after 10 years of chemical vs integrated fertilization. Chil. J. Agric. Res. 2017, 77, 355–364. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238–5259. [Google Scholar] [CrossRef] [PubMed]
- Abdul, N.S.; Marnewick, J.L. Fumonisin B1-induced mitochondrial toxicity and hepatoprotective potential of rooibos: An update. J. Appl. Toxicol. 2020, 40, 1602–1613. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. Eval. Carcinog. Risks Hum. 2002, 82, 1–556. [Google Scholar]
- Chu, F.S.; Li, G.Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 1994, 60, 847–852. [Google Scholar] [CrossRef]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef]
- Wall-Martínez, H.A.; Ramírez-Martínez, A.; Wesolek, N.; Brabet, C.; Durand, N.; Rodríguez-Jimenes, G.C.; García-Alvarado, M.A.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; Roudot, A.C. Risk assessment of exposure to mycotoxins (aflatoxins and fumonisins) through corn tortilla intake in veracruz city (Mexico). Food Addit. Contam. Part A 2019, 36, 929–939. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Zhang, M.; Otake, K.; Miyauchi, Y.; Yagi, M.; Yonei, Y.; Miyakawa, T.; Tanokura, M. Comprehensive NMR analysis of two kinds of post-fermented tea and their anti-glycation activities in vitro. Food Chem. 2019, 277, 735–743. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Shii, T.; Matsuo, Y.; Tanaka, T.; Jiang, Z.H.; Kouno, I. A new catechin oxidation product and polymeric polyphenols of post-fermented tea. Food Chem. 2011, 129, 830–8306. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aty, A.M.; Choi, J.H.; Rahman, M.M.; Kim, S.W.; Tosun, A.; Shim, J.H. Residues and contaminants in tea and tea infusions: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization Press: Geneva, Switzerland, 2011; pp. 146–160. [Google Scholar]
- Sedova, I.; Kiseleva, M.; Tutelyan, V. Mycotoxins in tea: Occurrence, methods of determination and risk evaluation. Toxins 2018, 10, 444. [Google Scholar] [CrossRef]
- Haas, D.; Pfeifer, B.; Reiterich, C.; Partenheimer, R.; Reck, B.; Buzina, W. Identification and quantification of fungi and mycotoxins from pu-erh tea. Int. J. Food Microbiol. 2013, 166, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Yan, H.; Granato, D.; Ho, C.T.; Ye, Z.; Wang, Y.; Zhang, L.; Zhou, Y. Quantitative analysis and dietary risk assessment of aflatoxins in Chinese post-fermented dark tea. Food Chem. Toxicol. 2020, 146, 111830. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, X.; Fu, R.; Yan, H.; Han, S.; Gerelt, K.; Cui, P.; Chen, J.; Qi, K.; Zhou, Y. Determination of six groups of mycotoxins in Chinese dark tea and the associated risk assessment. Environ. Pollut. 2020, 261, 114180. [Google Scholar] [CrossRef]
- Li, Z.; Mao, Y.; Teng, J.; Xia, N.; Huang, L.; Wei, B.; Chen, Q. Evaluation of mycoflora and citrinin occurrence in Chinese Liupao Tea. J. Agric. Food Chem. 2020, 68, 12116–12123. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Haughey, S.A.; Chevallier, O.P.; Galvin-King, P.; Campbell, K.; Magowan, E.; Adam, G.; Berthiller, F.; Krska, R.; Elliott, C.T. Determination of the mycotoxin content in distiller’ s dried grain with solubles using a multianalyte UHPLCMS/MS method. J. Agric. Food Chem. 2015, 63, 9441–9451. [Google Scholar] [CrossRef]
- Pantano, L.; La Scala, L.; Olibrio, F.; Galluzzo, F.G.; Bongiorno, C.; Buscemi, M.D.; Macaluso, A.; Vella, A. QuEChERS LC-MS/MS screening method for mycotoxin detection in cereal products and spices. Int. J. Environ. Res. Public. Health 2021, 18, 3774. [Google Scholar] [CrossRef]
- Mokubedi, S.M.; Phoku, J.Z.; Changwa, R.N.; Gbashi, S.; Njobeh, P.B. Analysis of mycotoxins contamination in poultry feeds manufactured in selected provinces of South Africa using UHPLC-MS/MS. Toxins 2019, 11, 452. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Abboodi, A.R.; Awawdeh, M.A.; Jbour, S.A.M.A. Affiliations expandassessment of mycotoxins (deoxynivalenol, zearalenone, aflatoxin B1 and fumonisin B1) in hen’s eggs in Jordan. Heliyon 2022, 8, e11017. [Google Scholar] [CrossRef]
- Wielogorska, E.; Mooney, M.; Eskola, M.; Ezekiel, C.N.; Stranska, M.; Krska, R.; Elliott, C. Occurrence and Human-Health impacts of mycotoxins in Somalia. J. Agric. Food Chem. 2019, 67, 2052–2060. [Google Scholar] [CrossRef]
- Kamala, A.; Kimanya, M.; Lachat, C.; Jacxsens, L.; Haesaert, G.; Kolsteren, P.; Ortiz, J.; Tiisekwa, B.; De Meulenaer, B. Risk of exposure to multiple mycotoxins from maize-based complementary foods in Tanzania. J. Agric. Food Chem. 2017, 65, 7106–7114. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Rocha, L.; Fontes, L.C.; Carnielli, L.; Reis, T.; Corrêa, B. Mycotoxin analysis of industrial beers from Brazil: The influence of fumonisin B1 and deoxynivalenol in beer quality. Food Chem. 2017, 218, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Sandoval, I.; Wesseling, S.; Rietjens, I.M.C.M. Occurrence and probabilistic risk assessment of fumonisin B1, fumonisin B2 and deoxynivalenol in nixtamalized maize in Mexico city. Toxins 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; RodríguezDelgado, M.Á. Evolution and applications of the QuEChERS method. Trac. Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Bordin, K.; Rottinghaus, G.E.; Landers, B.R.; Ledoux, D.R.; Kobashigawa, E.; Corassin, C.H.; Oliveira, C.A.F. Evaluation of fumonisin exposure by determination of fumonisin B1 in human hair and in Brazilian corn products. Food Control 2015, 53, 67–71. [Google Scholar] [CrossRef]
- Yapo, A.E.; Strub, C.; Durand, N.; Ahoua, A.R.C.; Schorr-Galindo, S.; Bonfoh, B.; Fontana, A.; Koussémon, M. Mass spectrometry-based detection and risk assessment of mycotoxin contamination of ‘kankankan’ used for roasted meat consumption in Abidjan, Côte d’Ivoire. Food Addit. Contam. 2020, 37, 1564–1578. [Google Scholar] [CrossRef]
- Visconti, A.; Doko, M.B.; Bottalico, C.; Schurer, B.; Boenke, A. Stability of fumonisins (FB1 and FB2) in solution. Food Addit. Contam. 1994, 11, 427–431. [Google Scholar] [CrossRef]
- Sulyok, M.; Krska, R.; Senyuva, H. Profiles of fungal metabolites including regulated mycotoxins in individual dried Turkish figs by LC-MS/MS. Mycotoxin Res. 2020, 36, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Du, L.J.; Chu, C.; Warner, E.; Wang, Q.Y.; Hu, Y.H.; Chai, K.J.; Cao, J.; Peng, L.Q.; Chen, Y.B.; Yang, J.; et al. Rapid microwave-assisted dispersive microsolid phase extraction of mycotoxins in food using zirconia nanoparticles. J. Chromatogr. A 2018, 1561, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, S.; Wu, A.; Zhang, D.; Van Peteghem, C.; De Saeger, S. Multimycotoxin UPLC−MS/MS for tea, herbal infusions and the derived drinkable products. J. Agric. Food Chem. 2010, 58, 12664–12671. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Gong, Y.Y.; Goycoolea, F.M. Aptamer-based detection of fumonisin B1: A critical review. Anal. Chim. Acta 2021, 1160, 338395–338427. [Google Scholar] [CrossRef]
- Dzuman, Z.; Zachariasova, M.; Veprikova, Z.; Godula, M.; Hajslova, J. Multi-analyte high-performance liquid chromatography coupled to high-resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta 2015, 863, 29–40. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No. 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. J. Eur. Communities 2006, 70, 12–34. [Google Scholar]
- Santos, L.; Marin, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multi contamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Omurtag, G.Z.; Yazicioglu, D. Determination of fumonisin B1 and B2 in herbal tea and medicinal plants in Turkey by high-performance liquid chromatography. J. Food Prot. 2004, 67, 1782–1786. [Google Scholar] [CrossRef]
- Martins, M.L.; Martins, H.M.; Bernardo, F. Fumonisins B1 and B2 in black tea and medicinal plants. J. Food Prot. 2001, 64, 1268–1270. [Google Scholar] [CrossRef]
- Wu, J.Y.; Yang, G.Y.; Chen, J.L.; Li, W.X.; Li, J.T.; Fu, C.X.; Jiang, G.F.; Zhu, W. Investigation for Pu-Erh Tea contamination caused by mycotoxins in a tea market in Guangzhou. J. Basic Appl. Sci. 2014, 10, 349–356. [Google Scholar]
- Nakagawa, H.; Hashimoto, R.; Matsuo, Y.; Sago, Y.; Yokoyama, K.; Takahashi, H. Detection and determination of fumonisins B1, B2, and B3 contaminating Japanese domestic wine by liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). Curr. Microbiol. 2022, 77, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.L.; Harris, C.A.; Clarke, R. An assessment of dietary exposure to glyphosate using refined deterministic and probabilistic methods. Food Chem. Toxicol. 2016, 95, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Chen, L.; Ju, S.Y.; Yang, H.; Zeng, Y.; Gu, D.; Ng, T.P. Type of tea consumption and depressive symptoms in Chinese older adults. BMC Geriatr. 2021, 21, 331. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Yang, J.F.; Xie, X.Y.; Lin, C.; Li, J.Y. Research on the behavior of tea consumption in China with the CKB data. J. Tea Sci. 2018, 38, 287–295. [Google Scholar]
- Jin, Y.L.; Liu, P.; Sun, J.F.; Wang, C.N.; Min, J.; Zhang, Y.F.; Wang, S.Y.; Wu, Y.N. Dietary exposure and risk assessment to lead of the population of Jiangsu province, China. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1187–1195. [Google Scholar] [CrossRef]
- Claeys, W.L.; Schmit, J.F.; Bragard, C.; Rogister, G.M.; Pussemier, L.; Schiffers, B. Exposure of several Belgian consumer groups to pesticide residues through fresh fruit and vegetable consumption. Food Control 2011, 22, 508–516. [Google Scholar] [CrossRef]
- GEMS/FOOD. Global environment monitoring system-food contamination monitoring and assessment programme. In GEM/Food Cluster Diets; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kiseleva, M.; Chalyy, Z.; Sedova, I. Tea: Transfer of mycotoxins from the spiked matrix into an infusion. Toxins 2021, 13, 404. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; Martínez, R.; González-Peñas, E.; Lizarraga, E.; Cerain, A.L. Co-occurrence of aflatoxins, ochratoxin a and zearalenone in breakfast cereals from Spanish market. Food Control 2011, 22, 1949–1955. [Google Scholar] [CrossRef]
- Li, W.G.; Xu, K.L.; Xiao, R.; Yin, G.F.; Liu, W.W. Development of an HPLC-based method for the detection of aflatoxins in Pu-erh tea. Int. J. Food Prop. 2015, 18, 842–848. [Google Scholar] [CrossRef]
- Pallarés, N.; Font, G.; Mañes, J.; Ferrer, E. Multimycotoxin LC-MS/MS analysis in tea beverages after dispersive liquid-liquid microextraction (DLLME). J. Agric. Food Chem. 2017, 65, 10282–10289. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.F.; Li, R.; Yang, H.; Qi, P.P.; Xiao, Y.P.; Qian, M.R. Occurrence of patulin in various fruit products and dietary exposure assessment for consumers in China. Food Control 2017, 78, 100–107. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin mycotoxin contamination in food and feed: Impact on agriculture, human health, and detection and management strategies. Toxins 2022, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Fusilier, K.; Chilvers, M.I.; Limay-Rios, V.; Singh, M.P. Mycotoxin co-occurrence in Michigan harvested maize grain. Toxins 2022, 24, 431. [Google Scholar] [CrossRef]
- Magembe, K.S.; Mwatawala, M.W.; Mamiro, D.P. Mycotoxin contamination in stored maize and groundnuts based on storage practices and conditions in subhumid tropical Africa: The case of Kilosa district, Tanzania. J. Food Prot. 2016, 79, 2160–2166. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Li, Y.; Geng, T.T.; Pan, X.F.; Zhou, Y.F.; Liu, G.; Pan, A. Overall lifestyles and socioeconomic inequity in mortality and life expectancy in China: The China health and nutrition survey. Age Ageing 2022, 51, 167. [Google Scholar] [CrossRef] [PubMed]
- Burstyn, I.; Teschke, K. Studying the determinants of ex-posure: A review of methods. Ann. Occup. Hyg. 1999, 60, 57–72. [Google Scholar]


| Mycotoxins | Fortification Level (μg/kg) | Recovery (%) | RSD (%) | LOQ (μg/kg) | LOD (μg/kg) | Regression Equation | R2 |
|---|---|---|---|---|---|---|---|
| Fumonisin B1 | 250 | 67.7 | 5.81 | 3 | 9 | y = 11,020x + 4705.7 | 0.9994 |
| Fumonisin B2 | 250 | 76.47 | 7.9 | 0.7 | 2 | y = 20,797x + 116,020 | 0.9989 |
| Fumonisin B3 | 62.5 | 77.13 | 5.78 | 0.7 | 2 | y = 26,700x + 53,722 | 0.9996 |
| PFT Products | Package Type | Positive/Total | Positive (%) | Number of Samples | FB1 Maximum (μg/kg) | FB2 Maximum (μg/kg) | FB1 M1 (μg/kg) | FB1 M2 (μg/kg) | FB2 M1 (μg/kg) | FB2 M2 (μg/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <LOD (μg/kg) | FB1 | FB2 | FB3 | FB1 and FB2 | ||||||||||
| Liupao teas | Basket packing, and paper packaging | 8/38 | 21.05 | 30 | 4 | 8 | 0 | 4 | 7.07 ± 0.24 | 11.00 ± 0.32 | 0.55 | 5.25 | 0.87 | 4.13 |
| Aluminum foil bag packaging, plastic bag packaging, and canned | 1/8 | 12.5 | 7 | 0 | 1 | 0 | 0 | / | 3.11 ± 0.14 | / | / | 0.39 | 3.11 | |
| Pu-erh teas | Basket packing, and paper packaging | 11/50 | 22 | 39 | 6 | 7 | 0 | 2 | 10.44 ± 0.11 | 15.00 ± 0.68 | 0.6 | 5 | 0.92 | 6.57 |
| Aluminum foil bag packaging, plastic bag packaging, and canned | 1/10 | 10 | 9 | 0 | 1 | 0 | 0 | / | 6.04 ± 0.78 | / | / | 0.6 | 6.04 | |
| Fu brick teas | Basket packing, and paper packaging | 3/14 | 21.43 | 11 | 2 | 3 | 0 | 2 | 11.81 ± 0.54 | 18.28 ± 0.34 | 1 | 7 | 1.86 | 8.67 |
| Total | 24/120 | 20 | 96 | 12 | 20 | 0 | 8 | 11.81 ± 0.54 | 18.28 ± 0.34 | 0.54 | 5.42 | 0.95 | 5.7 | |
| Demographic Characteristics | Population Ratio | |
|---|---|---|
| Gender | Male | 575 (63.18%) |
| Female | 335 (36.81%) | |
| Age | 18–44 | 372 (40.88%) |
| 45–60 | 437 (48.02%) | |
| >60 | 101 (11.10%) | |
| Respondents distribution | Nanning | 96 (10.55%) |
| Liuzhou | 174 (19.12%) | |
| Guilin | 228 (25.06%) | |
| Wuzhou | 100 (10.99%) | |
| Other cities | 312 (34.36%) | |
| Total | 910 (100%) | |
| Average body weight (kg) | 62.11 ± 11.01 | |
| PFT Products | Sample Population Ratio | Brewing Method | Sample Population Ratio | ||
|---|---|---|---|---|---|
| Tea preference | Liupao teas | 502 (55.16%) | Brewing method | Multiple brewing for drinking | 371 (40.77%) |
| Pu-erh teas | 197 (21.65%) | preference | Single brewing for drinking | 338 (36.70%) | |
| Fu brick teas | 41 (4.51%) | Long-term steaming for drinking | 141 (15.49%) | ||
| More than one type of tea | 170 (18.68%) | More than one brewing method | 64 (7.03%) | ||
| Times/day | Sample population ratio | Times/week | Sample population ratio | Times/month | Sample population ratio |
| 1–2 | 639 (70.22%) | 1–7 | 656 (72.09%) | 1–15 | 398 (43.74%) |
| 3–4 | 252 (27.70%) | 8–14 | 197 (21.65%) | 16–30 | 377 (41.43%) |
| >4 | 19 (2.09%) | >14 | 57 (6.26%) | >30 | 135 (14.84%) |
| PFT Products | FB Mean | FB Intake | HQ1 (%) | HQ2 (%) | HI (%) | ||
|---|---|---|---|---|---|---|---|
| FB1 Mean (μg/kg) | FB2 Mean (μg/kg) | FB1 Intake (μg/kg·bw/day) | FB2 Intake (μg/kg·bw/day) | ||||
| Liupao tea | 1.83 | 1 | 0.000274 | 0.00015 | 0.0137 | 0.00749 | 0.02119 |
| Pu-erh tea | 1.85 | 1.17 | 0.000278 | 0.000176 | 0.01388 | 0.00878 | 0.02265 |
| Fu brick tea | 2.29 | 2.13 | 0.000343 | 0.00032 | 0.01714 | 0.01599 | 0.03313 |
| Average | 1.99 | 1.43 | 0.000298 | 0.000215 | 0.0149 | 0.01075 | 0.02566 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, T.; Zhu, J.; Zhang, H.; Xu, B.; Guo, Y.; Li, J.; Xu, X.; Peng, F.; Liu, W.; Zhao, S.; et al. B-Type Fumonisins in Post-Fermented Tea: Occurrence and Consumer Dietary Exposure in Guangxi, China. Toxins 2023, 15, 534. https://doi.org/10.3390/toxins15090534
Qiu T, Zhu J, Zhang H, Xu B, Guo Y, Li J, Xu X, Peng F, Liu W, Zhao S, et al. B-Type Fumonisins in Post-Fermented Tea: Occurrence and Consumer Dietary Exposure in Guangxi, China. Toxins. 2023; 15(9):534. https://doi.org/10.3390/toxins15090534
Chicago/Turabian StyleQiu, Taotao, Jialin Zhu, Huayi Zhang, Biyun Xu, Yanju Guo, Jingrong Li, Xin Xu, Fenglin Peng, Weiguo Liu, Shengmei Zhao, and et al. 2023. "B-Type Fumonisins in Post-Fermented Tea: Occurrence and Consumer Dietary Exposure in Guangxi, China" Toxins 15, no. 9: 534. https://doi.org/10.3390/toxins15090534