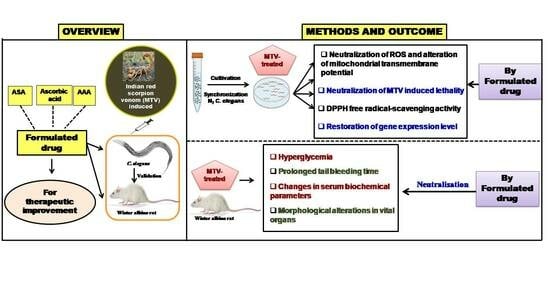
A Novel Therapeutic Formulation for the Improved Treatment of Indian Red Scorpion (Mesobuthus tamulus) Venom-Induced Toxicity-Tested in Caenorhabditis elegans and Rodent Models
Abstract
:1. Introduction
2. Results
2.1. The In-Silico Analysis Showed the Binding of AAAs to Homologous SER6 Receptors in C. elegans
2.2. Optimum Dose of Inhibitors in Neutralizing the MTV-Induced Lethality, ROS Generation, and Depolarization of Mitochondrial Transmembrane Potential in C. elegans
2.3. Early Treatment with AAAs, Commercial ASAs, and Ascorbic Acid Showed Better Neutralisation of MTV-Induced Toxicity in C. elegans
2.4. Formulated Drug Showed Significantly Higher Efficiency Compared to Individual Components of Formulation in Neutralising the MTV-Induced Lethality in C. elegans
2.5. The Formulated Drug (Formulation 2) Demonstrated Optimum Efficiency in Neutralising the In Vitro DPPH-Free Radical Scavenging Activity and In Vivo Neutralisation of MTV-Induced Reactive Oxygen Species (ROS) Generation and Alteration of Mitochondrial Transmembrane Potential (MMP) in C. elegans
2.6. Formulated Drug Restored the MTV-Induced Upregulation of Genes Involved in Apoptosis, Detoxification, and Stress Response to Delay MTV-Induced Programmed Cell Death in C. elegans
2.7. Neutralisation of MTV-Induced Hyperglycemia and Pathophysiological Symptoms, Prolonged Tail Bleeding Time, Serum Biochemical Changes, and Morphological Alterations in Wistar Strain Albino Rats Model by Drug Formulation 2
2.8. Decrease of Pro-Inflammatory Cytokines in MTV-Treated Swiss Albino Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Computational (In Silico) Analysis to Compare the Binding Efficiency of AAAs between α1-Adrenergic Receptor (α1A, α1B, and α1D) in Humans and Mice and Homologous Receptor (SER6) in C. elegans
5.2.1. Preparation of the Ligand 3D Structures for Docking
5.2.2. Protein-Ligand Docking
5.3. Determination of In Vivo Neutralisation Potency of Commercial ASAs, AAAs, and Ascorbic Acid in C. elegans Model
5.3.1. Cultivation and Synchronization N2 C. elegans Worms
5.3.2. Determination of Lethal Concentration 50 (LC50) of MTV in C. elegans
5.3.3. Determination of Dose- and Time-Dependent Neutralisation of MTV-Induced Toxicity in C. elegans by Commercial ASAs, AAAs, and Ascorbic Acid
5.3.4. In Vivo Neutralisation of MTV-Induced Generation of ROS and Alteration of MMP in C. elegans by ASA, AAAs, and Ascorbic Acid
5.4. The In Vivo Neutralisation of MTV-Induced Lethality in C. elegans with Individual Components of the Formulation and Their Combinations
5.5. In Vitro DPPH Free Radical-Scavenging Activity of Different Concentrations of the Formulated Drug, Individual Components of the Formulation, and Their Combinations
5.6. In Vivo Neutralisation of MTV-Induced Generation of ROS and Alteration MMP in C. elegans by Different Concentrations of the Formulated Drug, Individual Components of the Formulation, and Combination Thereof
5.7. Restoration of MTV-Induced Expression Level of Genes Involved in Apoptosis, Detoxification, and Stress Response by the Formulated Drug Was Determined by Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
5.8. Validation of In Vivo Neutralisation of MTV-Induced Toxicity by Formulated Drug and Combinations of Commercial ASA and AAA in Wistar Strain Albino Rats
5.8.1. Neutralisation of Hyperglycemia and Prolonged Tail Bleeding Time
5.8.2. Neutralisation of Changes in Serum Biochemical Parameters
5.8.3. Neutralisation of Morphological Alterations in Vital Organs
5.9. Determination of MTV-Induced Inflammatory Cytokines Levels in Swiss Albino Mice
5.10. Statistical Analysis
6. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bawaskar, H.; Bawaskar, P. Management of the cardiovascular manifestations of poisoning by the Indian red scorpion (Mesobuthus tamulus). Heart 1992, 68, 478–480. [Google Scholar] [CrossRef] [Green Version]
- Das, B.; Patra, A.; Mukherjee, A.K. Correlation of venom toxinome composition of Indian red scorpion (Mesobuthus tamulus) with clinical manifestations of scorpion stings: Failure of commercial antivenom to immune-recognize the abundance of low molecular mass toxins of this venom. J. Proteome Res. 2020, 19, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Saviola, A.J.; Mukherjee, A.K. Biochemical and proteomic characterization, and pharmacological insights of indian red scorpion venom toxins. Front. Pharmacol. 2021, 12, 710680. [Google Scholar] [CrossRef] [PubMed]
- Gwee, M.C.; Nirthanan, S.; Khoo, H.E.; Gopalakrishnakone, P.; Kini, R.M.; Cheah, L.S. Autonomic effects of some scorpion venoms and toxins. Clin. Exp. Pharmacol. Physiol. 2002, 29, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Ravens, U.; Cerbai, E. Role of potassium currents in cardiac arrhythmias. Europace 2008, 10, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Rowan, E.; Vatanpour, H.; Furman, B.; Harvey, A.; Tanira, M.; Gopalakrishnakone, P. The effects of Indian red scorpion Buthus tamulus venom in vivo and in vitro. Toxicon 1992, 30, 1157–1164. [Google Scholar] [CrossRef]
- Ruff, R.L. Slow Na+ channel inactivation must be disrupted to evoke prolonged depolarization-induced paralysis. Biophys. J. 1994, 66 Pt 1, 542. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.; Peigneur, S.; Tytgat, J. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2011, 2, 71. [Google Scholar] [CrossRef] [Green Version]
- Abroug, F.; Ouanes-Besbes, L.; Tilouche, N.; Elatrous, S. Scorpion envenomation: State of the art. Intensive Care Med. 2020, 46, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Cupo, P. Clinical update on scorpion envenoming. Rev. Soc. Bras. Med. Trop. 2015, 48, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Isbister, G.K.; Bawaskar, H.S. Scorpion envenomation. N. Engl. J. Med. 2014, 371, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Vatanpour, H.; Harvey, A.; Boyot, P.; Pinkasfeld, S.; Doljansky, Y.; Bouet, F.; Menez, A. Neuromuscular effects of some potassium channel blocking toxins from the venom of the scorpion Leiurus quinquestriatus hebreus. Toxicon 1994, 32, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Romey, G.; Abita, J.; Chicheportiche, R.; Rochat, H.; Lazdunski, M. Scorpion neurotoxin: Mode of action on neuromuscular junctions and synaptosomes. Biochim. Biophys. Acta-Biomembr. 1976, 448, 607–619. [Google Scholar] [CrossRef]
- Das, B.; Patra, A.; Puzari, U.; Deb, P.; Mukherjee, A.K. In vitro laboratory analyses of commercial anti-scorpion (Mesobuthus tamulus) antivenoms reveal their quality and safety but the prevalence of a low proportion of venom-specific antibodies. Toxicon 2022, 215, 37–48. [Google Scholar] [CrossRef] [PubMed]
- WHO. Snakebite Envenoming: A Strategy for Prevention and Control; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Bawaskar, H.S.; Bawaskar, P.H. Efficacy and safety of scorpion antivenom plus prazosin compared with prazosin alone for venomous scorpion (Mesobuthus tamulus) sting: Randomised open label clinical trial. BMJ 2011, 342, c7136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, C.; Caldara, R.; Ferrari, C.; Dal Bo, G.A.; Paracchi, A.; Romussi, M.; Curtarelli, G. Metabolic effects of prazosin. Clin. Pharmacol. Ther. 1980, 27, 313–316. [Google Scholar] [CrossRef]
- Murashita, M.; Kusumi, I.; Hosoda, H.; Kangawa, K.; Koyama, T. Acute administration of clozapine concurrently increases blood glucose and circulating plasma ghrelin levels in rats. Psychoneuroendocrinology 2007, 32, 777–784. [Google Scholar] [CrossRef]
- Bahloul, M.; Turki, O.; Chaari, A.; Bouaziz, M. Incidence, mechanisms and impact outcome of hyperglycaemia in severe scorpion-envenomed patients. Ther. Adv. Endocrinol. Metab. 2018, 9, 199–208. [Google Scholar] [CrossRef]
- Biswal, S.; Remick, D.G. Sepsis: Redox mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2007, 9, 1959–1962. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Jalali, M. Effect of vitamin C on Hemiscorpius lepturus scorpion venom on the level of some biochemical parameters in rats. J. Exp. Zool. 2010, 13, 573–579. [Google Scholar]
- Artal-Sanz, M.; de Jong, L.; Tavernarakis, N. Caenorhabditis elegans: A versatile platform for drug discovery. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 1405–1418. [Google Scholar] [CrossRef]
- Packham, R.; Walker, R.J.; Holden-Dye, L. The effect of a selective octopamine antagonist, epinastine, on pharyngeal pumping in Caenorhabditis elegans. Invertebr. Neurosci. 2010, 10, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balla, K.M.; Troemel, E.R. C aenorhabditis elegans as a model for intracellular pathogen infection. Cell. Microbiol. 2013, 15, 1313–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriadi, M.; Hart, A.C. Neurodegenerative disorders: Insights from the nematode Caenorhabditis elegans. Neurobiol. Dis. 2010, 40, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Ruszkiewicz, J.A.; Pinkas, A.; Miah, M.R.; Weitz, R.L.; Lawes, M.J.; Akinyemi, A.J.; Ijomone, O.M.; Aschner, M. C. elegans as a model in developmental neurotoxicology. Toxicol. Appl. Pharmacol. 2018, 354, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Nass, R.; Hamza, I. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Curr. Protoc. Toxicol. 2007, 31, 1.9.1–1.9.18. [Google Scholar] [CrossRef]
- Tralau, T.; Riebeling, C.; Pirow, R.; Oelgeschläger, M.; Seiler, A.; Liebsch, M.; Luch, A. Wind of change challenges toxicological regulators. Environ. Health Perspect. 2012, 120, 1489–1494. [Google Scholar] [CrossRef] [Green Version]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Kumar, J.; Park, K.-C.; Awasthi, A.; Prasad, B. Silymarin extends lifespan and reduces proteotoxicity in C. elegans Alzheimer’s model. CNS Neurol. Disord.-Drug Targets 2015, 14, 295–302. [Google Scholar] [CrossRef]
- Markaki, M.; Tavernarakis, N. Modeling human diseases in Caenorhabditis elegans. Biotechnol. J. 2010, 5, 1261–1276. [Google Scholar] [CrossRef]
- Cole, R.D.; Anderson, G.L.; Williams, P.L. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol. Appl. Pharmacol. 2004, 194, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Williams, P.L. Toxicity testing of neurotoxic pesticides in Caenorhabditis elegans. J. Toxicol. Environ. Health B 2014, 17, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Perez, D.M. α1-Adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Front. Pharmacol. 2020, 11, 581098. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Yoshida, M.; Oami, E.; Wang, M.; Ishiura, S.; Suo, S. Nonredundant function of two highly homologous octopamine receptors in food-deprivation-mediated signaling in Caenorhabditis elegans. J. Neurosci. Res. 2014, 92, 671–678. [Google Scholar] [CrossRef]
- Bargmann, C.I. Beyond the connectome: How neuromodulators shape neural circuits. Bioessays 2012, 34, 458–465. [Google Scholar] [CrossRef]
- Komuniecki, R.; Harris, G.; Hapiak, V.; Wragg, R.; Bamber, B. Monoamines activate neuropeptide signaling cascades to modulate nociception in C. elegans: A useful model for the modulation of chronic pain? Invertebr. Neurosci. 2012, 12, 53–61. [Google Scholar] [CrossRef]
- Bawaskar, H. Management of severe scorpion sting at rural settings: What is the role of scorpion antivenom? J. Venom. Anim. Toxins Incl. Trop. Dis. 2005, 11, 3–7. [Google Scholar] [CrossRef]
- Freire-Maia, L.; Campos, J.; Amaral, C. Approaches to the treatment of scorpion envenoming. Toxicon 1994, 32, 1009–1014. [Google Scholar] [CrossRef]
- Ismail, M. The scorpion envenoming syndrome. Toxicon 1995, 33, 825–858. [Google Scholar] [CrossRef]
- Alavi, S.M.; Azarkish, A. Secondary Bacterial Infection among the Patients with Scorpion Sting in Razi hospital, Ahvaz, Iran. Jundishapur J. Microbiol. 2011, 4, 37–42. [Google Scholar]
- MS, P. Cellulitis, necrotizing fasciitis, and subcutaneous tissue infections. Mand. Douglas Bennett’s Princ. Pract. Infect. Dis. 2010, 1, 1289–1312. [Google Scholar]
- Wheatley, G.H., III; Wait, M.A.; Jessen, M.E. Infective endocarditis associated with a scorpion sting. Ann. Thorac. Surg. 2005, 80, 1489–1490. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, M.; Bahloul, M.; Hergafi, L.; Kallel, H.; Chaari, L.; Ben Hamida, C.; Chaari, A.; Chelly, H.; Rekik, N. Factors associated with pulmonary edema in severe scorpion sting patients—A multivariate analysis of 428 cases. Clin. Toxicol. 2006, 44, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, M.; Bahloul, M.; Kallel, H.; Samet, M.; Ksibi, H.; Dammak, H.; Ahmed, M.N.B.; Chtara, K.; Chelly, H.; Hamida, C.B. Epidemiological, clinical characteristics and outcome of severe scorpion envenomation in South Tunisia: Multivariate analysis of 951 cases. Toxicon 2008, 52, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Yugandhar, B.; Radha Krishna Murthy, K.; Sattar, S. Insulin administration in severe scorpion envenoming. J. Venom. Anim. Toxins 1999, 5, 200–219. [Google Scholar] [CrossRef]
- Bawaskar, H.; Bawaskar, P. Utility of scorpion antivenin vs prazosin in the management of severe Mesobuthus tamulus (Indian red scorpion) envenoming at rural setting. J. Assoc. Physicians India 2007, 55, 14–21. [Google Scholar] [PubMed]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion venom causes apoptosis by increasing reactive oxygen species and cell cycle arrest in MDA-MB-231 and HCT-8 cancer cell lines. J. Evid. -Based Integr. Med. 2018, 23, 2156587217751796. [Google Scholar] [CrossRef] [Green Version]
- Bawaskar, H.; Bawaskar, P. Vasodilators: Scorpion envenoming and the heart (an Indian experience). Toxicon 1994, 32, 1031–1040. [Google Scholar] [CrossRef]
- Sofer, S.; Shahak, E.; Gueron, M. Scorpion envenomation and antivenom therapy. J. Pediatr. 1994, 124, 973–978. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Mackessy, S.P. Prevention and improvement of clinical management of snakebite in Southern Asian countries: A proposed road map. Toxicon 2021, 200, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K. Treatment of scorpion envenoming syndrome--need for scientific magnanimity. J. Indian Med. Assoc. 2013, 111, 254–259. [Google Scholar]
- Murthy, K.R.K.; Haghnazari, L. The blood levels of glucagon, cortisol and insulin following the injection of venom by the scorpion (Mesobuthus tamulus concanesis, Pocock) in dogs. J. Venom. Anim. Toxins Incl. Trop. Dis. 1999, 5, 47–55. [Google Scholar] [CrossRef]
- Mirakabbadi, A.Z.; Zolfagharian, H.; Hedayat, A.; Jalali, A. Clinical and biochemical manifestation produced by scorpion (Hemiscorpius lepturus) venom in experimental animals. J. Venom. Anim. Toxins Incl. Trop. Dis. 2007, 13, 758–765. [Google Scholar] [CrossRef]
- More, S.S.; Kiran, K.M.; Gadag, J. Dose-dependent serum biochemical alterations in Wistar albino rats after Palamneus gravimanus (Indian black scorpion) envenomation. J. Basic Clin. Physiol. Pharmacol. 2004, 15, 263–276. [Google Scholar] [CrossRef]
- Omran, M.A.; Abdel-Rahman, M.S. Effect of scorpion Leiurus quinquestriatus (H&E) venom on the clinical chemistry parameters of the rat. Toxicol. Lett. 1992, 61, 99–109. [Google Scholar] [PubMed]
- Ozkan, O.; Carhan, A. The neutralizing capacity of Androctonus crassicauda antivenom against Mesobuthus eupeus scorpion venom. Toxicon 2008, 52, 375–379. [Google Scholar] [CrossRef]
- Benvenuti, L.A.; Douetts, K.; Cardoso, J. Myocardial necrosis after envenomation by the scorpion Tityus serrulatus. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 275–276. [Google Scholar] [CrossRef]
- Köse, A.; Biricik, S.; Bozkurt, S.; Cavuşoğlu, C.; Ayrık, C. Toxic Hepatitis and Coagulopathy due to Scorpion Sting. Ann. Clin. Case Rep. 2016, 2016, 1212. [Google Scholar]
- Ismail, M.; Asaad, N.; Al Suwaidi, J.; Al Kawari, M.; Salam, A. Acute myocarditis and pulmonary edema due to scorpion sting. Glob. Cardiol. Sci. Pract. 2016, 2016, e201610. [Google Scholar] [CrossRef] [Green Version]
- Amaral, C.; Rezende, N. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon 1997, 35, 997–998. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.F.S.; de Rezende, N.A.; Freire-Maia, L. Acute pulmonary edema after Tityus serrulatus scorpion sting in children. Am. J. Cardiol. 1993, 71, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Sadowski, J.; Gasteiger, J.; Klebe, G. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J. Chem. Inf. Comput. Sci. 1994, 34, 1000–1008. [Google Scholar] [CrossRef]
- Schwab, C.H. Conformations and 3D pharmacophore searching. Drug Discov. Today Technol. 2010, 7, e245–e253. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Chem. Biol. Methods Protoc. 2015, 1263, 243–250. [Google Scholar]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Sulston, J.; Hodgkin, J. The Nematode Caenorhabditis elegans; Cold Spring Harbor Laboratory: New York, NY, USA, 1988. [Google Scholar]
- Puzari, U.; Fernandes, P.A.; Mukherjee, A.K. Advances in the therapeutic application of small-molecule inhibitors and repurposed drugs against snakebite: Miniperspective. J. Med. Chem. 2021, 64, 13938–13979. [Google Scholar] [CrossRef]
- Madhubala, D.; Patra, A.; Islam, T.; Saikia, K.; Khan, M.R.; Ahmed, S.A.; Borah, J.C.; Mukherjee, A.K. Snake venom nerve growth factor-inspired designing of novel peptide therapeutics for the prevention of paraquat-induced apoptosis, neurodegeneration, and alteration of metabolic pathway genes in the rat pheochromocytoma PC-12 cell. Free Radic. Biol. Med. 2023, 197, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Khadse, S. Scorpion Sting. J. Pediatr. Crit. Care 2016, 3, 42. [Google Scholar] [CrossRef]
- Sarma, P.; Bharadwaj, S.; Swargiary, D.; Ahmed, S.A.; Sheikh, Y.; Barge, S.R.; Manna, P.; Talukdar, N.C.; Bora, J.; Borah, J.C. Iridoid glycoside isolated from Wendlandia glabrata and the role of its enriched fraction in regulating AMPK/PEPCK/G6Pase signaling pathway of hepatic gluconeogenesis. New J. Chem. 2022, 46, 13167–13177. [Google Scholar] [CrossRef]
- Kiernan, J. Histopathological and Biochemical Methods: Theory and Practice, 3rd ed.; Butterworth Heinmann: Oxford, UK, 1999; pp. 154–155. [Google Scholar]















| S. No. | Components | Viability of C. elegans (%) | |
|---|---|---|---|
| 0 h | 24 h | ||
| 1 | Control (untreated) | 100.00 ± 5 | 96.30 ± 4.8 |
| 2 | MTV (LC50 value, 125 µg/mL) | 100.00 ± 5 | 51.92 ± 2.6 (Ψ) |
| 3 | MTV co-treated with ascorbic acid (0 min): ASA (60 min) | 100.00 ± 5 | 77.19 ± 3.8 (*) |
| 4 | MTV co-treated ascorbic acid (0 min): ASA (120 min) | 100.00 ± 5 | 69.49 ± 3.47 (*) |
| S. No. | Components | Viability of C. elegans (%) | |
|---|---|---|---|
| 0 h | 24 h | ||
| 1 | Control | 100.00 ± 5 | 91.5 ± 4.51 |
| 2 | MTV (LC50 value, 125 µg/mL) | 100.00 ± 5 | 51.6 ± 2.57 (Ψ) |
| 3 | MTV (LC50 value) pre-treated with ASA (187.5 µg) | 100.00 ± 5 | 59.5 ± 2.97 |
| 4 | MTV (LC50 value) treated with AAA (Prazosin, 3 µM) | 100.00 ± 5 | 64.4 ± 3.22 |
| 5 | MTV (LC50 value) treated with ascorbic acid (0.1 µg) | 100.00 ± 5 | 63.2 ± 3.16 |
| 6 | MTV (LC50 value) treated with ASA (187.5 µg): AAA (3 µM) | 100.00 ± 5 | 58.9 ± 2.94 |
| 7 | MTV treated with ascorbic acid (0.1 µg): AAA (3 µM) | 100.00 ± 5 | 63.2 ± 3.11 |
| 8 | MTV treated with ASA (187.5 µg): ascorbic acid (0.1 µg) | 100.00 ± 5 | 64.5 ± 3.22 |
| 9 | MTV treated with ASA (93.75 µg): ascorbic acid (0.05 µg): AAA (1.5 µM) [Formulation 1] | 100.00 ± 5 | 75.0 ± 3.75 |
| 10 | MTV treated with ASA (187.5 µg): ascorbic acid (0.1 µg): AAA (3 µM) [Formulation 2] | 100.00 ± 5 | 82.6 ± 4.13 (*) |
| 11 | MTV treated with ASA (375 µg): ascorbic acid (0.2 µg): AAA (6 µM) [Formulation 3] | 100.00 ± 5 | 89.1 ± 4.45 |
| S. No. | Components | ALKP Activity (U/L) | SGPT Activity (U/L) | Creatinine Activity (U/L) |
|---|---|---|---|---|
| 1 | Control | 108.00 ± 5.13 | 48.11 ± 2.40 | 0.27 ± 0.01 |
| 2 | MTV (LC50) | 180.50 ± 5.02 (Ψ) | 75.77 ± 3.78 (Ψ) | 0.44 ± 0.02 (Ψ) |
| 3 | ASA (1500 µg) | 122.20 ± 3.45 (*) | 39.90 ± 1.20 (*) | 0.31 ± 0.01 (*) |
| 4 | Ascorbic acid (1 µg) | 113.00 ± 5.31 (*) | 45.66 ± 2.28 (*) | 0.29 ± 0.01 (*) |
| 5 | AAA (50 µM) | 121.50 ± 4.56 (*) | 40.75 ± 2.03 (*) | 0.33 ± 0.02 (*) |
| 6 | ASA (1500 µg): AAA (50 µM) | 106.90 ± 4.96 (*) | 42.00 ± 2.13(*) | 0.30 ± 0.01 (*) |
| 7 | ASA (187.5 µg): AAA (3 µM) | 130.00 ± 3.12 (*) | 51.00 ± 2.55 (*) | 0.39 ± 0.01 (*) |
| 8 | Formulation 2 | 102.71 ± 4.20 (*) | 37.00 ± 1.85 (*) | 0.28 ± 0.01 (*) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.; Madhubala, D.; Mahanta, S.; Patra, A.; Puzari, U.; Khan, M.R.; Mukherjee, A.K. A Novel Therapeutic Formulation for the Improved Treatment of Indian Red Scorpion (Mesobuthus tamulus) Venom-Induced Toxicity-Tested in Caenorhabditis elegans and Rodent Models. Toxins 2023, 15, 504. https://doi.org/10.3390/toxins15080504
Das B, Madhubala D, Mahanta S, Patra A, Puzari U, Khan MR, Mukherjee AK. A Novel Therapeutic Formulation for the Improved Treatment of Indian Red Scorpion (Mesobuthus tamulus) Venom-Induced Toxicity-Tested in Caenorhabditis elegans and Rodent Models. Toxins. 2023; 15(8):504. https://doi.org/10.3390/toxins15080504
Chicago/Turabian StyleDas, Bhabana, Dev Madhubala, Saurov Mahanta, Aparup Patra, Upasana Puzari, Mojibur R. Khan, and Ashis K. Mukherjee. 2023. "A Novel Therapeutic Formulation for the Improved Treatment of Indian Red Scorpion (Mesobuthus tamulus) Venom-Induced Toxicity-Tested in Caenorhabditis elegans and Rodent Models" Toxins 15, no. 8: 504. https://doi.org/10.3390/toxins15080504