Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water
Abstract
:1. Introduction
1.1. Mycotoxins as Pollutants in Surface Waters
1.2. Analytical Methods for Zearalenone Determination
2. Results and Discussion
2.1. Determination of Zearalenone by Autofluorescence
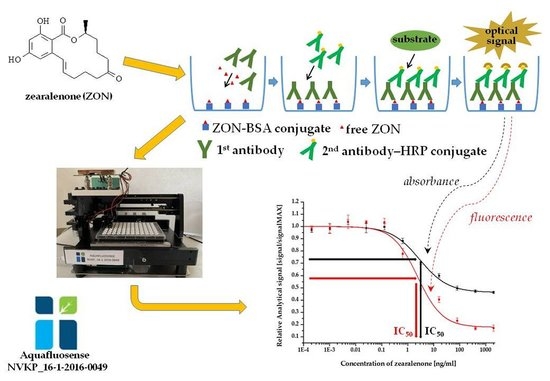
2.2. Enzyme-Linked Fluorescent Immunoassay (ELFIA)
2.2.1. Titration and Inhibition of the Antiserum
2.2.2. Immunoassay
2.2.3. Effects of Light Source Intensity
2.2.4. Cross-Reactivity of the Antisera with Zearalenone Derivatives
2.2.5. Analytical Detection Capability Compared to Other Immunoanalytical Methods
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Total Internal Reflection Ellipsometry
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Instrumentation
4.3. Determination of Zearalenone by Autofluorescence
4.4. Enzyme-Linked Immunofluorescence Assay
4.4.1. Hapten Synthesis and Conjugation
4.4.2. Serum Preparation
4.4.3. Immunoassay
4.5. High-Performance Liquid Chromatography (HPLC)
4.6. Total Internal Reflection Ellipsometry (TIRE)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- Krska, R.; Molinelli, A. Mycotoxin analysis: State-of-the-art and future trends. Anal. Bioanal. Chem. 2006, 387, 145–148. [Google Scholar] [CrossRef]
- Rubert, J.; Soriano, J.M.; Mañes, J.; Soler, C. Rapid mycotoxin analysis in human urine: A pilot study. Food Chem. Toxicol. 2011, 49, 2299–2304. [Google Scholar] [CrossRef]
- Van Der Fels-Klerx, H.; De Rijk, T.; Booij, C.; Goedhart, P.; Boers, E.; Zhao, C.; Waalwijk, C.; Mol, H.; Van Der Lee, T. Occurrence of Fusarium head blight species and Fusarium mycotoxins in winter wheat in the Netherlands in 2009. Food Addit. Contam. Part A 2012, 29, 1716–1726. [Google Scholar] [CrossRef]
- Valverde-Bogantes, E.; Bianchini, A.; Herr, J.R.; Rose, D.J.; Wegulo, S.N.; Hallen-Adams, H.E. Recent population changes of Fusarium head blight pathogens: Drivers and implications. Can. J. Plant Pathol. 2020, 42, 315–329. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Šegvić Klarić, M.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment changes, aflatoxins, and health issues, a review. Int. J. Environ. Res. Public Health 2020, 17, 7850. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J. Invited review: Diagnosis of zearalenone (ZEN) exposure of farm animals and transfer of its residues into edible tissues (carry over). Food Chem. Toxicol. 2015, 84, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.; Paterson, R.R.M. Comparisons of ergosterol to other methods for determination of Fusarium graminearum biomass in water as a model system. In Proceedings of the 22nd European Culture Collections’ Organization Meeting, Braga, Portugal, 17–19 September 2003; Lia, N., Smith, D., Eds.; Micoteca da Universidade do Minho: Braga, Portugal, 2003; pp. 235–239. Available online: https://repositorium.sdum.uminho.pt/bitstream/1822/55312/1/document_3404_1.pdf (accessed on 8 February 2021).
- Picardo, M.; Filatova, D.; Nuñez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. TrAC Trends Anal. Chem. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Russell, R.; Paterson, M. Zearalenone production and growth in drinking water inoculated with Fusarium graminearum. Mycol. Prog. 2007, 6, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Bucheli, T.D.; Wettstein, F.E.; Hartmann, N.; Erbs, M.; Vogelgsang, S.; Forrer, H.-R.; Schwarzenbach, R.P. Fusarium mycotoxins: Overlooked aquatic micropollutants? J. Agric. Food Chem. 2008, 56, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, K.; Waśkiewicz, A.; Goliński, P.; Świetlik, J. Occurrence of estrogenic mycotoxin—Zearalenone in aqueous environmental samples with various NOM content. Water Res. 2009, 43, 1051–1059. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Gromadzka, K.; Bocianowski, J.; Pluta, P.; Goliński, P. Zearalenone Contamination of the aquatic environment as a result of its presence in crops/Pojava mikotoksina u vodenom okolišu zbog njihove prisutnosti u usjevima. Arch. Ind. Hyg. Toxicol. 2012, 63, 429–435. [Google Scholar] [CrossRef]
- Jarošová, B.; Javůrek, J.; Adamovský, O.; Hilscherová, K. Phytoestrogens and mycoestrogens in surface waters—Their sources, occurrence, and potential contribution to estrogenic activity. Environ. Int. 2015, 81, 26–44. [Google Scholar] [CrossRef]
- Laranjeiro, C.S.; Da Silva, L.J.G.; Pereira, A.M.; Pena, A.; Lino, C.M. The mycoestrogen zearalenone in Portuguese flowing waters and its potential environmental impact. Mycotoxin Res. 2017, 34, 77–83. [Google Scholar] [CrossRef]
- Al-Gabr, H.M.; Zheng, T.; Yu, X. Fungi contamination of drinking water. Rev. Environ. Contam. Toxicol. 2013, 228, 121–139. [Google Scholar] [CrossRef]
- Hartmann, N.; Erbs, M.; Wettstein, F.E.; Schwarzenbach, R.P.; Bucheli, T.D. Quantification of estrogenic mycotoxins at the ng/L level in aqueous environmental samples using deuterated internal standards. J. Chromatogr. A 2007, 1138, 132–140. [Google Scholar] [CrossRef]
- Criado, M.V.; Pinto, V.E.F.; Badessari, A.; Cabral, D. Conditions that regulate the growth of moulds inoculated into bottled mineral water. Int. J. Food Microbiol. 2005, 99, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Fernandes, D.; Carvalho, G.; Benoliel, M.; Romão, M.S.; Crespo, M.B. Assessment of the presence and dynamics of fungi in drinking water sources using cultural and molecular methods. Water Res. 2010, 44, 4850–4859. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Schenzel, J.; Meyer, M.T.; Phillips, P.J.; Hubbard, L.E.; Scott, T.-M.; Bucheli, T.D. Mycotoxins: Diffuse and point source contributions of natural contaminants of emerging concern to streams. Sci. Total. Environ. 2014, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal contaminants in drinking water regulation? A tale of ecology, exposure, purification and clinical relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.; Kelley, J.; Gallagher, M. Natural occurrence of aflatoxins and Aspergillus flaws (Link) in water. Lett. Appl. Microbiol. 1997, 25, 435–436. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Mata, A.T.; Ferreira, J.P.; Crespo, M.T.B.; Pereira, V.J.; Bronze, M.R. Production of mycotoxins by filamentous fungi in untreated surface water. Environ. Sci. Pollut. Res. 2018, 25, 17519–17528. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Hoerger, C.C.; Meyer, M.T.; Wettstein, F.E.; Hubbard, L.E.; Bucheli, T.D. Phytoestrogens and Mycotoxins in Iowa Streams: An examination of underinvestigated compounds in agricultural basins. J. Environ. Qual. 2010, 39, 2089–2099. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.; Oliveira, B.; Batoréu, M.; Crespo, M.B.; Pereira, V.; Bronze, M. Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem. 2015, 176, 455–464. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Bocianowski, J.; Perczak, A.; Goliński, P. Occurrence of fungal metabolites—Fumonisins at the ng/L level in aqueous environmental samples. Sci. Total. Environ. 2015, 524-525, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Emídio, E.S.; Da Silva, C.P.; De Marchi, M.R.R. Estrogenic mycotoxins in surface waters of the Rico Stream micro-basin, São Paulo, Brazil: Occurrence and potential estrogenic contribution. Eclética Química J. 2020, 45, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Maragos, C.M. Zearalenone occurrence in surface waters in central Illinois, USA. Food Addit. Contam. Part B 2012, 5, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.; Erbs, M.; Forrer, H.-R.; Vogelgsang, S.; Wettstein, F.E.; Schwarzenbach, R.P.; Bucheli, T.D. Occurrence of Zearalenone on Fusarium graminearum infected wheat and maize fields in crop organs, soil, and drainage water. Environ. Sci. Technol. 2008, 42, 5455–5460. [Google Scholar] [CrossRef]
- Laganà, A.; Bacaloni, A.; De Leva, I.; Faberi, A.; Fago, G.; Marino, A. Analytical methodologies for determining the occurrence of endocrine disrupting chemicals in sewage treatment plants and natural waters. Anal. Chim. Acta 2004, 501, 79–88. [Google Scholar] [CrossRef]
- Pietsch, C. Zearalenone (ZEN) and its influence on regulation of gene expression in carp (Cyprinus carpio L.) liver tissue. Toxins 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khezri, A.; Herranz-Jusdado, J.G.; Ropstad, E.; Fraser, T.W. Mycotoxins induce developmental toxicity and behavioural aberrations in zebrafish larvae. Environ. Pollut. 2018, 242, 500–506. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- European Commission. Commission regulation (EC) No 1881/2006 of 19 December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364–365. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; DiNovi, M.; Edler, L.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, e04851. [Google Scholar] [CrossRef] [Green Version]
- Caglayan, M.O.; Şahin, S.; Üstündağ, Z. Detection strategies of zearalenone for food safety: A review. Crit. Rev. Anal. Chem. 2020, 67, 1–20. [Google Scholar] [CrossRef]
- Turner, G.V.; Phillips, T.D.; Heidelbaugh, N.D.; Russell, L.H. High Pressure liquid chromatographic determination of zearalenone in chicken tissues. J. Assoc. Off. Anal. Chem. 1983, 66, 102–104. [Google Scholar] [CrossRef]
- Sebaei, A.S.; Gomaa, A.M.; Mohamed, G.G.; El-Di, F.N. Simple validated method for determination of deoxynivalenol and zearalenone in some cereals using high performance liquid chromatography. Am. J. Food Technol. 2012, 7, 668–678. [Google Scholar] [CrossRef]
- Majerus, P.; Graf, N.; Krämer, M. Rapid determination of zearalenone in edible oils by HPLC with fluorescence detection. Mycotoxin Res. 2009, 25, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M.; Lawrence, J.W.; van Walbeek, W. Detection of mycotoxins by thin-layer chromatography: Application to screening of fungal extracts. App. Environ. Microbiol. 1970, 20, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Larionova, D.A.; Goryacheva, I.Y.; Van Peteghem, C.; De Saeger, S. Thin-layer chromatography of aflatoxins and zearalenones with β-cyclodextrins as mobile phase additives. World Mycotoxin J. 2011, 4, 113–117. [Google Scholar] [CrossRef]
- Soares, L.M.V. Multi-toxin TLC methods for aflatoxins, ochratoxin a, zearalenone and sterigmatocystin in foods. In Analysis of Plant Waste Materials; Springer International Publishing: Geneva, Switzerland, 1992; Volume 13, pp. 227–238. [Google Scholar]
- Thouvenot, D.; Morfin, R. Quantitation of zearalenone by gas-liquid chromatography on capillary glass columns. J. Chromatogr. A 1979, 170, 165–173. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-based liquid chromatography-tandem mass spectrometry method for the simultaneous determination of nine zearalenone-like mycotoxins in pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the multi-mycotoxin determination in composite cereal-based samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Jodlbauer, J.; Zöllner, P.; Lindner, W. Determination of zeranol, taleranol, zearalenone, α- and β-zearalenol in urine and tissue by high-performance liquid chromatography-tandem mass spectrometry. Chromatographia 2000, 51, 681–687. [Google Scholar] [CrossRef]
- Pestka, J.J.; Abouzied, M.N. Immunological assays for mycotoxin detection. Food Technol. 1995, 49, 120–128. [Google Scholar]
- Maragos, C.M.; Kim, E.-K. Detection of zearalenone and related metabolites by fluorescence polarization immunoassay. J. Food Prot. 2004, 67, 1039–1043. [Google Scholar] [CrossRef]
- Hao, K.; Suryoprabowo, S.; Song, S.; Liu, L.; Kuang, H. Rapid detection of zearalenone and its metabolite in corn flour with the immunochromatographic test strip. Food Agric. Immunol. 2017, 29, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Renaud, J.B.; Miller, J.D.; Sumarah, M.W. Mycotoxin testing paradigm: Challenges and opportunities for the future. J. AOAC Int. 2019, 102, 1681–1688. [Google Scholar] [CrossRef]
- Dong, G.; Pan, Y.; Wang, Y.; Ahmed, S.; Liu, Z.; Peng, D.; Yuan, Z. Preparation of a broad-spectrum anti-zearalenone and its primary analogues antibody and its application in an indirect competitive enzyme-linked immunosorbent assay. Food Chem. 2018, 247, 8–15. [Google Scholar] [CrossRef]
- Liu, M.T.; Ram, B.P.; Hart, L.P.; Pestka, J.J. Indirect enzyme-linked immunosorbent assay for the mycotoxin zearalenone. Appl. Environ. Microbiol. 1985, 50, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Nie, D.; Zhao, Z.; Meng, X.; Wu, A. Ultrasensitive immunoassays based on biotin–streptavidin amplified system for quantitative determination of family zearalenones. Food Control. 2015, 57, 202–209. [Google Scholar] [CrossRef]
- Pichler, H.; Krska, R.; Szekacs, A.; Grasserbauer, M. An enzyme-immunoassay for the detection of the mycotoxin zearalenone by use of yolk antibodies. Anal. Bioanal. Chem. 1998, 362, 176–177. [Google Scholar] [CrossRef]
- Radová, Z.; HajšLová, J.; Králová, J.; Papoušková, L.; Sýkorová, S. Analysis of Zearalenone in wheat using high-performance liquid chromatography with fluorescence detection and/or enzyme-linked immunosorbent assay. Cereal Res. Commun. 2001, 29, 435–442. [Google Scholar] [CrossRef]
- Tang, X.; Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Ding, X.; Lei, J.; Zhang, Z. Development and application of an immu-noaffinity column enzyme immunoassay for mycotoxin zearalenone in complicated samples. PLoS ONE 2014, 9, e85606. [Google Scholar]
- Thongrussamee, T.; Kuzmina, N.; Shim, W.-B.; Jiratpong, T.; Eremin, S.; Intrasook, J.; Chung, D.-H. Monoclonal-based enzyme-linked immunosorbent assay for the detection of zearalenone in cereals. Food Addit. Contam. Part A 2008, 25, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Thouvenot, D.; Morfin, R.F. Radioimmunoassay for zearalenone and zearalanol in human serum: Production, properties, and use of porcine antibodies. Appl. Environ. Microbiol. 1983, 45, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Chun, H.S.; Choi, E.H.; Chang, H.-J.; Choi, S.-W.; Eremin, S.A. A fluorescence polarization immunoassay for the detection of zearalenone in corn. Anal. Chim. Acta 2009, 639, 83–89. [Google Scholar] [CrossRef]
- Van Der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; Van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control. 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Goud, Y.K.; Kumar, S.V.; Hayat, K.; Gobi, V.K.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Maragos, C.M. Gold nanoparticle-enhanced multiplexed imaging surface plasmon resonance (iSPR) detection of Fusarium mycotoxins in wheat. Biosens. Bioelectron. 2018, 101, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nabok, A.; Al-Jawdah, A.; Gémes, B.; Takács, E.; Székács, A. An optical planar waveguide-based immunosensors for determination of Fusarium mycotoxin zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Székács, I.; Adányi, N.; Szendrő, I.; Székács, A. Direct and Competitive optical grating immunosensors for determination of Fusarium mycotoxin zearalenone. Toxins 2021, 13, 43. [Google Scholar] [CrossRef]
- Urraca, J.L.; Benito-Peña, E.; Pérez-Conde, C.; Moreno-Bondi, M.C.; Pestka, J.J. Analysis of zearalenone in cereal and swine feed samples using an automated flow-through immunosensor. J. Agric. Food Chem. 2005, 53, 3338–3344. [Google Scholar] [CrossRef]
- Aquafluosense. komplex vízminősítést in situ megvalósító, közvetlen és immunfluoreszcencián, valamint optikai és lézeres plazma-színképelemzésen alapuló, moduláris, érzékelő- és műszercsalád kifejlesztése, továbbá az alkalmazási területek kutatása. Available online: http://aquafluosense.hu (accessed on 8 February 2021).
- Rodbard, D.; Hutt, D.M. Statistical analysis of radioimmunoassays and immunoradiometric (labeled antibody) assays: A generalized, weighted, iterative, least-squares method for logistic curve fitting. In Proceedings of the Symposium on RIA and Related Procedures in Medicine, Proceedings of the Internationaé Atomic Energy Agency, Vienna, Austria, 1974; p. 165. [Google Scholar]
- Miles, C.O.; Erasmuson, A.F.; Wilkins, A.L.; Towers, N.R.; Smith, B.L.; Garthwaite, I.; Scahill, B.G.; Hansen, R.P. Ovine metabolism of zearalenone to α-zearalanol (Zeranol). J. Agric. Food Chem. 1996, 44, 3244–3250. [Google Scholar] [CrossRef]
- Megharaj, M.; Garthwaite, I.; Thiele, J.H. Total biodegradation of the oestrogenic mycotoxin zearalenone by a bacterial culture. Lett. Appl. Microbiol. 1997, 24, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Nabok, A.; Tsargorodskaya, A. The method of total internal reflection ellipsometry for thin film characterisation and sensing. Thin Solid Film. 2008, 516, 8993–9001. [Google Scholar] [CrossRef]
- Csősz, D.; Lenk, S.; Barócsi, A.; Csőke, T.L.; Klátyik, S.; Lázár, D.; Berki, M.; Adányi, N.; Csákányi, A.; Domján, L.; et al. Sensitive fluorescence instrumentation for water quality assessment. In Proceedings of the Optical Sensors and Sensing Congress (ES, FTS, HISE, Sensors), San Jose, CA, USA, 25–27 June 2019; OSA: Washington, DC, USA, 2019. [Google Scholar]






| Equation for fitting: 1 | ||
| Adjusted R2: 0.990 (absorbance) 0.988 (fluorescence) | ||
| Parameter | Value ± Standard Deviation | |
| Absorbance | 0.98 ± 0.02 | |
| 0.45 ± 0.01 | ||
| 2.86 ± 0.38 | ||
| 0.79 ± 0.12 | ||
| Fluorescence | 0.99 ± 0.01 | |
| 0.16 ± 0.03 | ||
| 2.41 ± 0.27 | ||
| 0.83 ± 0.19 | ||
| Mycotoxin | Detection Mode | |||
|---|---|---|---|---|
| Fluorescence | Absorbance | |||
| IC50 (ng/mL) 1 | CR% 2 | IC50 (ng/mL) | CR% 1 | |
| zearalenone | 2.20 ± 0.31 | 100 | 2.73 ± 0.35 | 100 |
| α-zearalenol | 10.42 ± 0.24 | 21.1 ± 3.0 | 10.94 ± 1.28 | 20.1 ± 2.6 |
| β-zearalenol | 8.74 ± 0.90 | 25.2 ± 3.6 | 8.65 ± 0.84 | 25.4 ± 3.3 |
| zearalanone | 8.56 ± 0.74 | 25.7 ± 3.6 | 8.24 ± 0.82 | 26.7 ± 3.4 |
| α-zearalanol | 35.36 ± 2.86 | 6.2 ± 0.9 | 35.63 ± 3.05 | 6.2 ± 0.8 |
| β-zearalanol | 200.7 ± 12.32 | 1.1 ± 0.2 | 250.02 ± 21.68 | 0.9 ± 0.1 |
| Analytical Method | LOD 1 (ng/mL) | IC50 2 Detection Range (ng/mL) | Matrix | Organic Solvent Content in the Sample Extract | Reference |
|---|---|---|---|---|---|
| ELISA 3 | 10 | 40 10–200 | maize | 10% AcCN 4 | [57] |
| Radioimmunoassay | 5 | NR 5 0.25–10 | human serum | - | [61] |
| ELISA | 1 | 3 0.5–50 | wheat, maize | 10% MeOH 6 | [55] |
| SPR (Sensor) 7 | 0.56 | 5 | wheat | 16% MeOH | [65] |
| DPV (Sensor) 8 | 0.25 | NR | beer, wine | 20% AcCN | [64] |
| ELISA | 0.24 | 0.855 9 | maize | 14% MeOH | [54] |
| ELISA | 0.15 (PBS) 0.23 (maize) | 1.13 (PBS) 1.4 (maize) | maize | 8% MeOH | [60] |
| FLISA 10 | 0.10 | 0.95 | maize flour | 14% MeOH | [56] |
| ELFIA 11 | 0.09 | 2.4 | water | 0.2% MeOH | this study |
| ELISA | 0.05 | NR | wheat | 10% MeOH | [58] |
| ELISA | 0.02 | 0.18 | maize flour | 14% MeOH | [56] |
| SPR (Sensor) | 0.01 | NR | NR | 9% AcCN | [63] |
| PW PI (Sensor) 12 | 0.01 | NR | water | 10% MeOH | [66] |
| CPG-Based Immunosensor 13 | 0.007 | 0.087 | wheat | 0.21% MeOH; 0.2% AcCN | [68] |
| ELISA (Coupled with IAC) 14 | 0.002 | 0.02 | maize | 10% AcCN | [59] |
| OWLS (Sensor) 15 | 2 × 10−6 | 0.014 | maize | AcCN | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gémes, B.; Takács, E.; Gádoros, P.; Barócsi, A.; Kocsányi, L.; Lenk, S.; Csákányi, A.; Kautny, S.; Domján, L.; Szarvas, G.; et al. Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water. Toxins 2021, 13, 182. https://doi.org/10.3390/toxins13030182
Gémes B, Takács E, Gádoros P, Barócsi A, Kocsányi L, Lenk S, Csákányi A, Kautny S, Domján L, Szarvas G, et al. Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water. Toxins. 2021; 13(3):182. https://doi.org/10.3390/toxins13030182
Chicago/Turabian StyleGémes, Borbála, Eszter Takács, Patrik Gádoros, Attila Barócsi, László Kocsányi, Sándor Lenk, Attila Csákányi, Szabolcs Kautny, László Domján, Gábor Szarvas, and et al. 2021. "Development of an Immunofluorescence Assay Module for Determination of the Mycotoxin Zearalenone in Water" Toxins 13, no. 3: 182. https://doi.org/10.3390/toxins13030182