Evaluation of Palm Oil as a Suitable Vegetable Oil for Vitamin A Fortification Programs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Oil, Chemicals and Materials
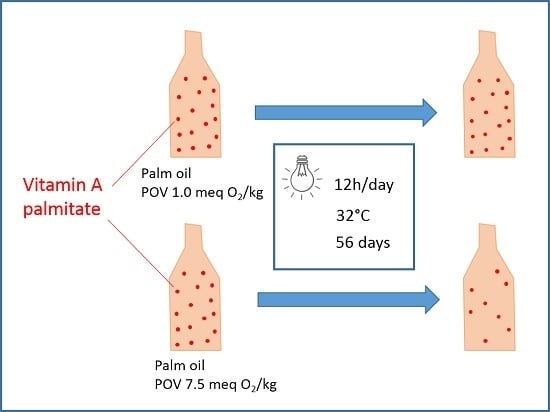
2.2. Study Design
2.3. Determination of the POV
2.4. Quantitation of the Fatty Acid Composition
2.5. Quantitation of Hexanal
2.6. Quantitation of Vitamin E
2.7. Quantitation and Decomposition of Vitamin A
2.8. Statistical Analyses
3. Results
3.1. Oxidative Stability of Mildly and Highly Oxidized Palm Oil Fortified with RP as Determined by the Fatty Acid Profile
3.2. Oxidative Stability of Mildly and Highly Oxidized Palm Oil Fortified with RP as Determined by the POV
3.3. Oxidative Stability of Mildly and Highly Oxidized Palm Oil Fortified with RP as Determined by a Secondary Lipid Oxidation Marker
3.4. Oxidative Stability of Mildly and Highly Oxidized Palm Oil Fortified with RP as Determined by the Vitamin E Content
3.5. Effect of the Oxidative Status of Palm Oil on the Stability of Vitamin A
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DIN | German Institute of Standardization |
| LOD | limit of detection |
| LOQ | limit of quantitation |
| meq | milliequivalents |
| POV | peroxide value |
| RP | retinyl palmitate |
References
- Ross, A.C. Vitamin A. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A., Caballero, B., Cousins, R., Tucker, K., Ziegler, T., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 260–277. [Google Scholar]
- Green, H.N.; Mellanby, E. Vitamin A as an anti-infective agent. Br. Med. J. 1928, 1928, 691–696. [Google Scholar] [CrossRef]
- Semba, R.D.; Bloem, M.W. The anemia of vitamin A deficiency: Epidemiology and pathogenesis. Eur. J. Clin. Nutr. 2002, 56, 271–281. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.M.; Roop, D.; Huang, F.L. Vitamin A: A key nutrient for the maintenance of epithelial differentiation. Acta Vitaminol. Enzymol. 1985, 7, 13–20. [Google Scholar] [PubMed]
- Akhtar, S.; Ahmed, A.; Randhawa, M.A.; Atukorala, S.; Arlappa, N.; Ismail, T.; Ali, Z. Prevalence of vitamin A deficiency in south asia: Causes, outcomes, and possible remedies. J. Health Popul Nutr. 2013, 31, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Englberger, L.; Darnton-Hill, I.; Coyne, T.; Fitzgerald, M.H.; Marks, G.C. Carotenoid-rich bananas: A potential food source for alleviating vitamin A deficiency. Food Nutr. Bull. 2003, 24, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Solomons, N.W.; Orozco, M. Alleviation of vitamin A deficiency with palm fruit and its products. Asia Pac. J. Clin. Nutr. 2003, 12, 373–384. [Google Scholar] [PubMed]
- WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005; Who Global Database on Vitamin A Deficiency: Geneva, Switzerland, 2009. [Google Scholar]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015, 3, e528–e536. [Google Scholar] [CrossRef]
- Sommer, A.; Alnwick, D.; Arthur, P.; Bloem, M.; Csete, J.; Dalmiya, N.; Davidson, F.; de Benoist, B.; Dwivedi, A.; Greene, J.; et al. Vitamin A global initiative. In A Strategy for Acceleration of Progress in Combating Vitamin A Deficiency; Consensus of an Informal Technical Consultation; Convened by the United Nations Children’s Fund (UNICEF) in Association with: The Micronutrient Initiative (MI, Ottawa, Canada), The World Health Organization (WHO, Geneva, Switzerland), The Canadian International Development Agency (CIDA, Quebec, Canada), The United States Agency for International Development (USAID, Washington, DC, USA); 1997. [Google Scholar]
- Kapil, U.; Tyagi, M. Scientific rationale for targeted vitamin A supplementation to children in India. Ind. J. Community Health 2011, 23, 1–3. [Google Scholar]
- Madatuwa, T.M.; Mahawithanage, S.T.; Chandrika, U.G.; Jansz, E.R.; Wickremasinghe, A.R. Evaluation of the effectiveness of the national vitamin A supplementation programme among school children in Sri Lanka. Br. J. Nutr. 2007, 97, 153–159. [Google Scholar] [CrossRef] [PubMed]
- De Moura, F.F.; Palmer, A.C.; Finkelstein, J.L.; Haas, J.D.; Murray-Kolb, L.E.; Wenger, M.J.; Birol, E.; Boy, E.; Pena-Rosas, J.P. Are biofortified staple food crops improving vitamin A and iron status in women and children? New evidence from efficacy trials. Adv. Nutr. 2014, 5, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Anjum, F.M.; Anjum, M.A. Micronutrient fortification of wheat flour: Recent development and strategies. Food Res. Int. 2011, 44, 652–659. [Google Scholar] [CrossRef]
- Dary, O. Sugar Fortification with Vitamin A: A Central American Contribution to the Developing World; Food Fortification to End Micronutrient Malnutrition: Ottawa, ON, Canada; The Micronutrient Initiative, International Development Research Centre: Ottawa, ON, Canada, 1997; pp. 95–98. [Google Scholar]
- Johnson, L.E. Oils, Fats and Margarine: Overview of Technology; Food Fortification to End Micronutrient Malnutrition: Ottawa, ON, Canada; The Micronutrient Initiative, International Development Research Centre: Ottawa, ON, Canada, 1997; pp. 22–26. [Google Scholar]
- Dary, O.; Mora, J.O. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. J. Nutr. 2002, 132, 2927S–2933S. [Google Scholar] [PubMed]
- Laillou, A.; Panagides, D.; Garrett, G.S.; Moench-Pfanner, R. Vitamin A-fortified vegetable oil exported from Malaysia and Indonesia can significantly contribute to vitamin A intake worldwide. Food Nutr. Bull. 2013, 34, S72–S80. [Google Scholar] [CrossRef] [PubMed]
- Laillou, A.; Hafez, S.A.; Mahmoud, A.H.; Mansour, M.; Rohner, F.; Fortin, S.; Berger, J.; Ibrahim, N.A.; Moench-Pfanner, R. Vegetable oil of poor quality is limiting the success of fortification with vitamin A in Egypt. Food Nutr. Bull. 2012, 33, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Dumhart, B.; Gartner, S.; Jirsa, F.; Steiger, G.; Kraemer, K.; Somoza, V. Vitamin A is rapidly degraded in retinyl palmitate-fortified soybean oil stored under household conditions. J. Agric. Food Chem. 2014, 62, 7559–7566. [Google Scholar] [CrossRef] [PubMed]
- Firestone, D. Method CD 8–53, 4th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Pignitter, M.; Stolze, K.; Gartner, S.; Dumhart, B.; Stoll, C.; Steiger, G.; Kraemer, K.; Somoza, V. Cold fluorescent light as major inducer of lipid oxidation in soybean oil stored at household conditions for eight weeks. J. Agric. Food Chem. 2014, 62, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Bognar, A. Bestimmung von Vitamin A in Lebensmitteln mittels Hochleistungs-flüssigchromatographie (HPLC). Z. Lebensm. Unters. Forsch. 1986, 182, 492–497. [Google Scholar] [CrossRef] [PubMed]
- USAID. Usaid’s Vitamin A Program: Ending Vitamin A Deficiency Worldwide 1965–1998; United States Agency For International Development: Washington, DC, USA, 1998.
- Lin, S.W. Palm Oil, 2nd ed.; Blackwell Publishing Ltd.: Oxford, UK, 2011; pp. 25–58. [Google Scholar]
- Cunnane, S.; Drevon, C.A.; Harris, B.; Sinclair, A.; Spector, A. Recommendations for Dietary Intake of Polyunsaturated Fatty Acids in Healthy Adults; International Society for the Study of Fatty Acids and Lipids: Washington, DC, USA, 2004. [Google Scholar]
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2012, 20, 772–777. [Google Scholar]
- FAO. Joint Fao/Who Codex Alimentarius Commission; Codex Alimentarius Commission: Rome, Italy, 1992. [Google Scholar]
- Ames, B.N. Dietary carcinogens and anticarcinogens-oxygen radicals and degenerative diseases. Science 1983, 221, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.S. Oxidized fat in the diet, postprandial lipaemia and cardiovascular disease. Curr. Opin. Lipidol. 2002, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Staprans, I.; Rapp, J.H.; Pan, X.M.; Hardman, D.A.; Feingold, K.R. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. [Breakdown of linoleic and linolenic acid hydroperoxides in the presence of ascorbic acid analysis of the volatile aldehydes (author’s transl)]. Z. Lebensm. Unters. Forsch. 1977, 163, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Player, M.E.; Kim, H.J.; Lee, H.O.; Min, D.B. Stability of alpha-, gamma-, or delta-tocopherol during soybean oil oxidation. J. Food Sci. 2006, 71, C456–C460. [Google Scholar] [CrossRef]
- Isnardy, B.; Wagner, K.H.; Elmadfa, I. Effects of alpha, gamma, and delta-tocopherols on the autoxidation of purified rapeseed oil triacylglycerols in a system containing low oxygen. J. Agric. Food Chem. 2003, 51, 7775–7780. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Hemery, Y.M.; Fontan, L.; Moench-Pfanner, R.; Laillou, A.; Berger, J.; Renaud, C.; Avallone, S. Influence of light exposure and oxidative status on the stability of vitamins A and D(3) during the storage of fortified soybean oil. Food Chem. 2015, 184, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Andarwulan, N.; Gitapratiwi, D.; Laillou, A.; Fitriani, D.; Hariyadi, P.; Moench-Pfanner, R.; Martianto, D. Quality of vegetable oil prior to fortification is an important criteria to achieve a health impact. Nutrients 2014, 6, 5051–5060. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]






| LOD (µg/mL) | LOQ (µg/mL) | |
|---|---|---|
| Methyl myristate | 0.004 | 0.013 |
| Methyl palmitate | 0.072 | 0.240 |
| Methyl palmitoleate | 0.005 | 0.017 |
| Methyl stearate | 0.028 | 0.093 |
| Methyl oleate | 0.098 | 0.327 |
| Methyl linoleate | 0.013 | 0.043 |
| Methyl linolenate | 0.005 | 0.017 |
| Fatty Acid | Peroxide value < 2 | Peroxide value > 5 | ||||
|---|---|---|---|---|---|---|
| Day 1 (mg/mL) | Day 57 (mg/mL) | Change (%) | Day 1 (mg/mL) | Day 57 (mg/mL) | Change (%) | |
| C14:0 | 8.61 ± 0.41 | 8.34 ± 0.45 | 2.97 ± 5.04 | 8.56 ± 0.27 | 8.71 ± 0.40 | −1.86 ± 5.69 |
| C16:0 | 484 ± 25.0 | 464 ± 24.6 | 3.98 ± 5.75 | 479 ± 23.8 | 489 ± 23.3 | −4.12 ± 6.25 |
| C16:1 | 2.08 ± 0.08 | 2.05 ± 0.07 | 1.62 ± 3.74 | 2.08 ± 0.04 | 2.13 ± 0.09 | −2.54 ± 4.85 |
| C18:0 | 62.9 ± 3.25 | 60.5 ± 3.03 | 3.55 ± 5.54 | 62.2 ± 2.45 | 63.6 ± 2.93 | −2.30 ± 6.13 |
| C18:1 | 535 ± 26.6 | 514 ± 26.1 | 3.88 ± 5.77 | 529 ± 21.6 | 540 ± 25.9 | −2.15 ± 6.32 |
| C18:2 | 108 ± 5.20 | 102 ± 4.97 | 5.54 ± 5.27 | 105 ± 4.78 | 106 ± 5.06 | −2.93 ± 5.88 |
| C18:3 | 3.13 ± 0.10 | 2.86 ± 0.11 | 8.45 ± 6.22 | 2.93 ± 0.10 | 2.92 ± 0.13 | 0.20 ± 5.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignitter, M.; Hernler, N.; Zaunschirm, M.; Kienesberger, J.; Somoza, M.M.; Kraemer, K.; Somoza, V. Evaluation of Palm Oil as a Suitable Vegetable Oil for Vitamin A Fortification Programs. Nutrients 2016, 8, 378. https://doi.org/10.3390/nu8060378
Pignitter M, Hernler N, Zaunschirm M, Kienesberger J, Somoza MM, Kraemer K, Somoza V. Evaluation of Palm Oil as a Suitable Vegetable Oil for Vitamin A Fortification Programs. Nutrients. 2016; 8(6):378. https://doi.org/10.3390/nu8060378
Chicago/Turabian StylePignitter, Marc, Natalie Hernler, Mathias Zaunschirm, Julia Kienesberger, Mark Manuel Somoza, Klaus Kraemer, and Veronika Somoza. 2016. "Evaluation of Palm Oil as a Suitable Vegetable Oil for Vitamin A Fortification Programs" Nutrients 8, no. 6: 378. https://doi.org/10.3390/nu8060378