Nutritional Intake and Status of Cobalamin and Folate among Non-Pregnant Women of Reproductive Age in Bhaktapur, Nepal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Population
2.2. Methods and Study Design
2.3. Ethical Approval
2.4. Blood Sampling and Processing
2.5. Statistical Analysis
2.6. Definitions
3. Results
3.1. General Characteristics
3.2. Cobalamin Intake
3.3. Folate Intake
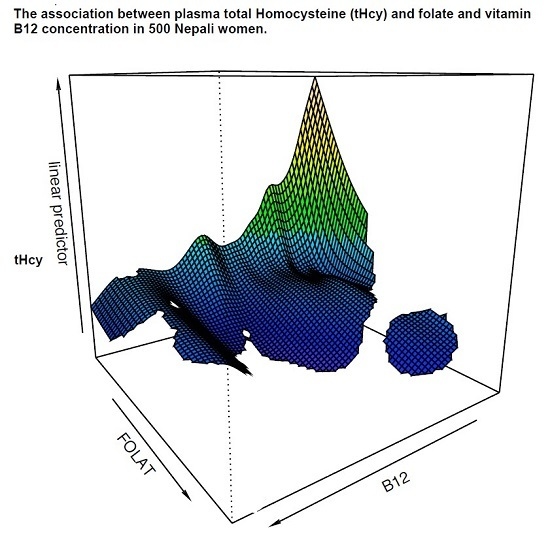
3.4. Plasma Cobalamin, Folate, MMA, and Hcyβ
3.5. Relationship between Intake and Status of Cobalamin and Folate
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MMA | methylmalonic acid |
| Hcy | plasma total homocysteine |
| FFQ | food frequency questionnaire |
| RDA | Recommended Dietary Allowances |
References
- Dror, D.K.; Allen, L.H. Effect of vitamin b12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr. Rev. 2008, 66, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Jernigan, T.L. The basics of brain development. Neuropsychol. Rev. 2010, 20, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Kirke, P.N.; Troendle, J.F.; Burke, H.; Sutton, M.; Brody, L.C.; Scott, J.M.; Mills, J.L. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 2009, 123, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Bhate, V.; Deshpande, S.; Bhat, D.; Joshi, N.; Ladkat, R.; Watve, S.; Fall, C.; de Jager, C.A.; Refsum, H.; Yajnik, C. Vitamin B12 status of pregnant Indian women and cognitive function in their 9-year-old children. Food Nutr. Bull. 2008, 29, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J. Nutr. 2010, 140, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Kurpad, A.V.; Duggan, C.P.; Bosch, R.J.; Dwarkanath, P.; Mhaskar, A.; Mhaskar, R.; Thomas, A.; Vaz, M.; Bhat, S.; et al. Low maternal vitamin b12 status is associated with intrauterine growth retardation in urban South Indians. Eur. J. Clin. Nutr. 2006, 60, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 2041–2042. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Refsum, H.; Beresford, S.A.; Vollset, S.E. The controversy over homocysteine and cardiovascular risk. Am. J. Clin. Nutr. 2000, 72, 324–332. [Google Scholar] [PubMed]
- Nelen, W.L.; Blom, H.J.; Steegers, E.A.; den Heijer, M.; Thomas, C.M.; Eskes, T.K. Homocysteine and folate levels as risk factors for recurrent early pregnancy loss. Obstet. Gynecol. 2000, 95, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Eskes, T.K. Clotting disorders and placental abruption: Homocysteine—A new risk factor. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 206–212. [Google Scholar] [CrossRef]
- Hogeveen, M.; Blom, H.J.; den Heijer, M. Maternal homocysteine and small-for-gestational-age offspring: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Duggan, C.; Thomas, T.; Bosch, R.; Rajendran, R.; Virtanen, S.M.; Srinivasan, K.; Kurpad, A.V. Vitamin B(12) intake and status in early pregnancy among urban south indian women. Ann. Nutr. Metab. 2013, 62, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Bondevik, G.T.; Schneede, J.; Refsum, H.; Lie, R.T.; Ulstein, M.; Kvale, G. Homocysteine and methylmalonic acid levels in pregnant nepali women. Should cobalamin supplementation be considered? Eur. J. Clin. Nutr. 2001, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Herbert, V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am. J. Clin. Nutr. 1994, 59, 1213S–1222S. [Google Scholar] [PubMed]
- Varela-Moreiras, G.; Murphy, M.M.; Scott, J.M. Cobalamin, folic acid, and homocysteine. Nutr. Rev. 2009, 67, S69–S72. [Google Scholar] [CrossRef] [PubMed]
- Casterline, J.E.; Allen, L.H.; Ruel, M.T. Vitamin B-12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J. Nutr. 1997, 127, 1966–1972. [Google Scholar] [PubMed]
- Barbosa, P.R.; Stabler, S.P.; Machado, A.L.; Braga, R.C.; Hirata, R.D.; Hirata, M.H.; Sampaio-Neto, L.F.; Allen, R.H.; Guerra-Shinohara, E.M. Association between decreased vitamin levels and MTHFR, MTR and MTRR gene polymorphisms as determinants for elevated total homocysteine concentrations in pregnant women. Eur. J. Clin. Nutr. 2008, 62, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Srinivasan, K.; Thomas, T.; Samuel, T.; Rajendran, R.; Muthayya, S.; Finkelstein, J.L.; Lukose, A.; Fawzi, W.; Allen, L.H.; et al. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J. Nutr. 2014, 144, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Siddiqua, T.J.; Ahmad, S.M.; Ahsan, K.B.; Rashid, M.; Roy, A.; Rahman, S.M.; Shahab-Ferdows, S.; Hampel, D.; Ahmed, T.; Allen, L.H.; et al. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: A randomized clinical trial in bangladesh. Eur. J. Nutr. 2016, 55, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Christian, P.; Khatry, S.K.; Wu, L.; West, K.P., Jr. Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J. Nutr. 2005, 135, 1106–1112. [Google Scholar] [PubMed]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P., Jr. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: The pune maternal nutrition study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Milman, N.; Byg, K.E.; Bergholt, T.; Eriksen, L.; Hvas, A.M. Cobalamin status during normal pregnancy and postpartum: A longitudinal study comprising 406 Danish women. Eur. J. Haematol. 2006, 76, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Fernandez-Ballart, J.D. Homocysteine in pregnancy. Adv. Clin. Chem. 2011, 53, 105–137. [Google Scholar] [PubMed]
- Abbenhardt, C.; Miller, J.W.; Song, X.; Brown, E.C.; Cheng, T.Y.; Wener, M.H.; Zheng, Y.; Toriola, A.T.; Neuhouser, M.L.; Beresford, S.A.; et al. Biomarkers of one-carbon metabolism are associated with biomarkers of inflammation in women. J. Nutr. 2014, 144, 714–721. [Google Scholar] [CrossRef] [PubMed]
- CBS. National Population and Housing Census; Central bureau of statistics (CBS); Government of Nepal; National Planning Commission Secretariat: Kathmandu, Nepal, 2011.
- Chandyo, R.K.; Strand, T.A.; Mathisen, M.; Ulak, M.; Adhikari, R.K.; Bolann, B.J.; Sommerfelt, H. Zinc deficiency is common among healthy women of reproductive age in Bhaktapur, Nepal. J. Nutr. 2009, 139, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Chandyo, R.K.; Strand, T.A.; Ulvik, R.J.; Adhikari, R.K.; Ulak, M.; Dixit, H.; Sommerfelt, H. Prevalence of iron deficiency and anemia among healthy women of reproductive age in Bhaktapur, Nepal. Eur. J. Clin. Nutr. 2007, 61, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Husek, P. Simultaneous profile analysis of plasma amino and organic acids by capillary gas chromatography. J. Chromatogr. B Biomed. Appl. 1995, 669, 352–357. [Google Scholar] [CrossRef]
- Moller, J.; Rasmussen, K.; Christensen, L. External quality assessment of methylmalonic acid and total homocysteine. Clin. Chem. 1999, 45, 1536–1542. [Google Scholar] [PubMed]
- Molloy, A.M.; Scott, J.M. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997, 281, 43–53. [Google Scholar] [PubMed]
- Kelleher, B.P.; Walshe, K.G.; Scott, J.M.; O’Broin, S.D. Microbiological assay for vitamin B12 with use of a colistin-sulfate-resistant organism. Clin. Chem. 1987, 33, 52–54. [Google Scholar] [PubMed]
- Hannisdal, R.; Ueland, P.M.; Svardal, A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin Chem. 2009, 147, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wfood2. World Food 2 Computer Software Package; The regents of the University of California: Berkeley, CA, USA, 1996. [Google Scholar]
- World Health Organization. Estimating Appropriate Levels of Vitamins and Minerals for Food Fortification Programs: The WHO Intake Monitoring, Assessment and Planning Program (IMAPP); Meeting Report; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef] [PubMed]
- CDC/MMWR. CDC Criteria for Anemia in Children and Child Bearing Aged Women; CDC Morbidity and Mortality Weekly Report; Centers for Disease Control: Atlanta, GA, USA, 1989; Volume 38, pp. 400–404. [Google Scholar]
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Population; Vitamin and Mineral Nutrition Information System; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Refsum, H.; Yajnik, C.S.; Gadkari, M.; Schneede, J.; Vollset, S.E.; Orning, L.; Guttormsen, A.B.; Joglekar, A.; Sayyad, M.G.; Ulvik, A.; et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am. J. Clin. Nutr. 2001, 74, 233–241. [Google Scholar] [PubMed]
- Hirsch, S.; de la Maza, P.; Barrera, G.; Gattas, V.; Petermann, M.; Bunout, D. The Chilean flour folic acid fortification program reduces serum homocysteine levels and masks vitamin B-12 deficiency in elderly people. J. Nutr. 2002, 132, 289–291. [Google Scholar] [PubMed]
- WHO/FAO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- ICMR. Nutrient Requirements and Recommended Dietary Allowances for Indians; Indian Council for Medical Research (ICMR); National Nutrition Institute: Hyderabad, India, 2010. [Google Scholar]
- Ulak, M.; Chandyo, R.K.; Adhikari, R.K.; Sharma, P.R.; Sommerfelt, H.; Refsum, H.; Strand, T.A. Cobalamin and folate status in 6 to 35 months old children presenting with acute diarrhea in Bhaktapur, Nepal. PLoS ONE 2014, 9, e90079. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Vikram, N.K.; Pandey, R.M.; Dwivedi, M.; Ahmad, F.U.; Luthra, K.; Jain, K.; Khanna, N.; Devi, J.R.; Sharma, R.; et al. Hyperhomocysteinemia, and low intakes of folic acid and vitamin B12 in urban North India. Eur. J. Nutr. 2002, 41, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Mallidi, P.V.; Vinarskiy, S.; Brar, S.; Frouhar, Z. Hyperhomocysteinemia and cobalamin deficiency in young Asian Indians in the united states. Am. J. Hematol. 2002, 70, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.J.; Huang, P.C.; Lu, S.C.; Li, Y.H.; Huang, H.B.; Lin, B.F.; Chang, S.J.; Chou, H.F. Plasma homocysteine levels in Taiwanese vegetarians are higher than those of omnivores. J. Nutr. 2002, 132, 152–158. [Google Scholar] [PubMed]
- Lindgren, A. Elevated serum methylmalonic acid. How much comes from Cobalamin deficiency and how much comes from the kidneys? Scand. J. Clin. Lab. Investig. 2002, 62, 15–19. [Google Scholar] [CrossRef]
- Joshi, D.D.; Maharjan, M.; Johansen, M.V.; Willingham, A.L.; Sharma, M. Improving meat inspection and control in resource-poor communities: The Nepal example. Acta Trop. 2003, 87, 119–127. [Google Scholar] [CrossRef]
- Albert, M.J.; Mathan, V.I.; Baker, S.J. Vitamin B12 synthesis by human small intestinal bacteria. Nature 1980, 283, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Dhonukshe-Rutten, R.A.; van Wijngaarden, J.P.; Zwaluw, N.L.; Velde, N.; de Groot, L.C. Dietary sources of vitamin B-12 and their association with vitamin B-12 status markers in healthy older adults in the B-PROOF study. Nutrients 2015, 7, 7781–7797. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Food and Agricultural Organization (FAO) and World Health Organization (WHO) Expert Consultation on Human Vitamin and Mineral Requirements; FAO/WHO: Washington, DC, USA, 2000. [Google Scholar]
- Bondevik, G.T.; Ulstein, M.; Lie, R.T.; Rana, G.; Kvale, G. The prevalence of anemia in pregnant Nepali women—A study in Kathmandu. Acta Obstet. Gynecol. Scand. 2000, 79, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Steegers-Theunissen, R.P.; Boers, G.H.; Trijbels, F.J.; Eskes, T.K. Neural-tube defects and derangement of homocysteine metabolism. N. Engl. J. Med. 1991, 324, 199–200. [Google Scholar] [PubMed]
- Scott, J.M.; Weir, D.G. The methyl folate trap. A physiological response in man to prevent methyl group deficiency in kwashiorkor (methionine deficiency) and an explanation for folic-acid induced exacerbation of subacute combined degeneration in pernicious anemia. Lancet 1981, 2, 337–340. [Google Scholar] [CrossRef]
- Dwarkanath, P.; Barzilay, J.R.; Thomas, T.; Thomas, A.; Bhat, S.; Kurpad, A.V. High folate and low vitamin b-12 intakes during pregnancy are associated with small-for-gestational age infants in South Indian women: A prospective observational cohort study. Am. J. Clin. Nutr. 2013, 98, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Bjorkegren, K.; Svardsudd, K. Serum cobalamin, folate, methylmalonic acid and total homocysteine as vitamin B12 and folate tissue deficiency markers amongst elderly swedes—A population-based study. J. Intern. Med. 2001, 249, 423–432. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | % (n) |
|---|---|
| Mean age (SD), y | 23 (6) |
| Parity one or more, n (%) 1 | 54 (268) |
| Mean age of menarche (SD), y | 14.6 (1.5) |
| Number of women using contraceptives 2 | 74 (219) |
| Number of women who smokes 3 | 6.6 (26) |
| Number of vegetarians 3 | 2 (10) |
| Mean family size (SD) | 7 (3) |
| Number of women working only in agriculture | 23 (117) |
| Number of women working on daily wage basis | 49 (247) |
| Mean body mass index, BMI (SD), kg/m2 | 21.8 (3.0) |
| Number of women with BMI <18.5 kg/m2 | 11 (55) |
| Mean Hemoglobin (SD), g/dL | 13.2 (1.2) |
| Total number of women with Hb <12.3 g/dL | 16 (79) |
| Number of women who cannot read/write | 33 (166) |
| Indicators | Values |
|---|---|
| Dietary intake (n = 379) | |
| Energy, kcal (median, IQR) | 1894 (1576, 2184) |
| % consuming <2200 Kcal, % (n) 1 | 76 (289) |
| Folate, µg (median, IQR) | 211 (130, 327) |
| % consuming <320 µg | 74 (279) |
| % consuming <200 µg | 47 (178) |
| Cobalamin, µg (median, IQR) | 0.34 (0.08, 1.1) |
| % consuming <2 µg | 88 (333) |
| % consuming <1 µg | 72 (271) |
| Biochemical markers (n = 500) | |
| Homocysteine (Hcy), µmol/L, mean (SD) | 9.0 (7.4, 11.4) |
| % of Hcy >7.5 µmol/L | 72 (361) |
| % of Hcy >10 µmol/L | 36 (182) |
| % of Hcy >15 µmol/L | 10 (52) |
| Folate, nmol/L (mean,SD) | 22.4 (17.1) |
| % of plasma folate <6.8 nmol/L | 4.6 (23) |
| % of plasma folate <10 nmol/L & Hcy >7.5 µmol/L | 4 (22) |
| Methylmalonic acid (MMA), µmol/L (mean, SD) | 0.62 (0.58) |
| % of MMA >0.26 µmol/L | 82 (405) |
| Cobalamin, pmol/L (mean,SD) | 173.2 (74.4) |
| % of plasma cobalamin <150 pmol/L | 42 (210) |
| % of plasma cobalamin <150 pmol/L & MMA >0.26 µmol/L | 37 (185) |
| % of plasma cobalamin <150 pmol/L & Hcy >7.5 µmol/L | 34 (170) |
| % of plasma cobalamin <150 pmol/L & plasma folate <6.8 nmol/L | 2 (10) |
| Frequency of Consumption | ||||
|---|---|---|---|---|
| Foods | Contribution (%) | Number of Recalls with Particular Food (%) | Number of Women Who Reported to Consumption Atleast a Week 2 % (n) | Content (µg) Per 100 gr Food 3 |
| Cobalamin | ||||
| Buffalo meat | 58% | 18 | 40 (159) | 1.47 |
| Milk (buffalo) 4 | 23% | 61 | 63 (247) | 0.46 |
| Eggs | 9% | 9 | 33 (130) | 1.1 |
| Goat meat | 4% | 1 | 1 (4) | 1.52 |
| Curds (yogurt) | 4% | 4 | 3 (12) | 0.37 |
| Fish | 1% | 0.5 | 1 (4) | 2.32 |
| Poultry | 1% | 3 | 7 (28) | 0.25 |
| Folate | ||||
| Green or dry leafy | 54% | 57 | 81 (319) | 118–146 |
| vegetables | ||||
| Rice | 8% | 100 | 100 (394) | 5.8 |
| Pulses/Beans | 7% | 61 | 67 (264) | 182 |
| (lentil, gram, peas) | ||||
| Wheat flour or grain | 5% | 41 | 65 (256) | 30 |
| Potato | 4% | 73 | 88 (347) | 9 |
| Rice flakes | 2% | 33 | 33 (130) | 12 |
| Variables | n (%) | Plasma Cobalamin | Plasma Folate | Plasma Methylmalonic Acid | Plasma Homocystiene |
|---|---|---|---|---|---|
| Age <20 years | 500 | ||||
| No | 328 (65.5) | ||||
| Yes | 172 (34.4) | (−18.5; −32.8, −4.2) 1 | (−4.2; −7.6, −0.88) | (0.15; 0.04, 0.27) | (0.87; −0.36, 2.1) |
| Working in carpet factories | 500 | ||||
| No | 407 (81.4) | ||||
| Yes | 93 (18.6) | (37.5; 19.6, 55.4) | (−4.5; −8.7, −0.34) | (−0.11; −0.25, 0.03) | (−0.05; −1.6. 1.5) |
| Illiterate mother | 497 | ||||
| No | 331 (66.6) | ||||
| Yes | 166 (33.4) | (4.5; −10.5, 19.5) | (−0.53; −4.1, 3.0) | (0.15; 0.03, 0.27) | (−0.52, −1.8, 0.76) |
| BMI <18.5 kg/m2 | 500 | ||||
| No | 445 (89) | ||||
| Yes | 55 (11) | (3.2; −17.5, 23.9) | (−1.7; −6.6, 3.1) | (0.03; −0.13, 0.19) | (0.23; −1.5, 1.9) |
| Atleat once a week meat intake 2 | 394 | ||||
| No | 234 (59.5) | ||||
| Yes | 160 (40.5) | (30.5; 15.8, 45.1) | (−0.36; −4.1, 3.4) | (−0.19; −0.3, −0.09) | (0.43; −1.7, 0.89) |
| Anemia (Hb <12.3 g/dL) | 500 | ||||
| No | 421 (84.2) | ||||
| Yes | 79 (15.8) | (6.4; −12.1, 24.9) | (0.42; −3.9, 4.8) | (−0.11; −0.25, 0.04) | (−0.45; −2.0, 1.1) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandyo, R.K.; Ulak, M.; Sommerfelt, H.; Schneede, J.; Ueland, P.M.; Strand, T.A. Nutritional Intake and Status of Cobalamin and Folate among Non-Pregnant Women of Reproductive Age in Bhaktapur, Nepal. Nutrients 2016, 8, 375. https://doi.org/10.3390/nu8060375
Chandyo RK, Ulak M, Sommerfelt H, Schneede J, Ueland PM, Strand TA. Nutritional Intake and Status of Cobalamin and Folate among Non-Pregnant Women of Reproductive Age in Bhaktapur, Nepal. Nutrients. 2016; 8(6):375. https://doi.org/10.3390/nu8060375
Chicago/Turabian StyleChandyo, Ram K., Manjeswori Ulak, Halvor Sommerfelt, Jørn Schneede, Per M. Ueland, and Tor A. Strand. 2016. "Nutritional Intake and Status of Cobalamin and Folate among Non-Pregnant Women of Reproductive Age in Bhaktapur, Nepal" Nutrients 8, no. 6: 375. https://doi.org/10.3390/nu8060375