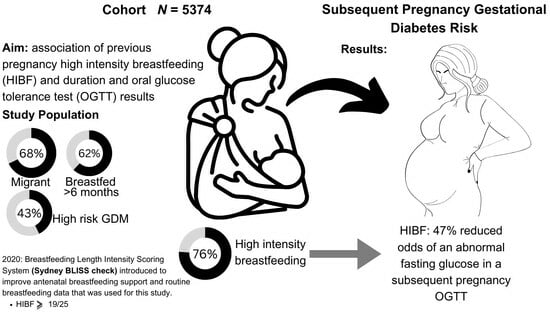
Previous High-Intensity Breastfeeding Lowers the Risk of an Abnormal Fasting Glucose in a Subsequent Pregnancy Oral Glucose Tolerance Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measures and Data Source
2.2. Breastfeeding Measures
2.3. Gestational Diabetes Measures
2.4. Body Mass Index Measure (BMI)
2.5. Demographic Measures
2.6. Statistical Analysis
3. Results
3.1. Cohort Characteristics and Exposure
3.2. Primary Aim: Association between Breastfeeding and OGTT Results in a Subsequent Pregnancy
3.3. Secondary Aim: Factors Associated with Both Reduced High-Intensity Breastfeeding Postpartum and Breastfeeding >6 Months
3.4. Factors Only Associated with Breastfeeding >6 Months
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunderson, E.P.; Jacobs Jr, D.R.; Chiang, V.; Lewis, C.E.; Feng, J.; Quesenberry, C.P., Jr.; Sidney, S. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes 2010, 59, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Tschiderer, L.; Seekircher, L.; Kunutsor, S.K.; Peters, S.A.E.; O’Keeffe, L.M.; Willeit, P. Breastfeeding Is Associated with a Reduced Maternal Cardiovascular Risk: Systematic Review and Meta-Analysis Involving Data From 8 Studies and 1 192 700 Parous Women. J. Am. Heart Assoc. 2022, 11, e022746. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Guideline. Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Smith, J.P. “Lost milk?”: Counting the economic value of breast milk in gross domestic product. J. Hum. Lact. 2013, 29, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.D.; Phan, L.T.H.; Mathisen, R. The cost of not breastfeeding: Global results from a new tool. Health Policy Plan. 2019, 34, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.; Vaz, J.S.; Maia, F.S.; Baker, P.; Gatica-Domínguez, G.; Piwoz, E.; Rollins, N.; Victora, C.G. Rates and time trends in the consumption of breastmilk, formula, and animal milk by children younger than 2 years from 2000 to 2019: Analysis of 113 countries. Lancet Child Adolesc. Health 2021, 5, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.W.; Jiang, S.; Athayde, N. Impact of the IADPSG criteria for gestational diabetes, and of obesity, on pregnancy outcomes. Aust. N. Z. J. Obstet. Gynaecol. 2018, 58, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Gandevani, S.; Amiri, M.; Bidhendi Yarandi, R.; Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2019, 11, 11. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef]
- Publications UN. The Sustainable Development Goals Report 2021; Jensen, L., Ed.; Department of Economic and Social Affairs: New York, NY, USA, 2021. [Google Scholar]
- Fu, J.; Retnakaran, R. The life course perspective of gestational diabetes: An opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine 2022, 45, 101294. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, M.; Viana, L.V.; Kramer, C.K. Lifestyle intervention for the prevention of type 2 diabetes in women with prior gestational diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2023, 25, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Lewis, C.E.; Lin, Y.; Sorel, M.; Gross, M.; Sidney, S.; Jacobs, D.R.; Shikany, J.M.; Quesenberry, C.P. Lactation Duration and Progression to Diabetes in Women Across the Childbearing Years: The 30-Year CARDIA Study. JAMA Intern. Med. 2018, 178, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, E.P.; Hedderson, M.M.; Chiang, V.; Crites, Y.; Walton, D.; Azevedo, R.A.; Fox, G.; Elmasian, C.; Young, S.; Salvador, N.; et al. Lactation intensity and postpartum maternal glucose tolerance and insulin resistance in women with recent GDM: The SWIFT cohort. Diabetes Care 2012, 35, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Leng, J.; Li, W.; Zhang, S.; Liu, H.; Shao, P.; Wang, P.; Wang, L.; Tian, H.; Zhang, C.; et al. Lactation intensity and duration to postpartum diabetes and prediabetes risk in women with gestational diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3115. [Google Scholar] [CrossRef]
- Melov, S.J.; White, L.; Simmons, M.; Kirby, A.; Stulz, V.; Padmanabhan, S.; Alahakoon, T.I.; Pasupathy, D.; Cheung, N.W. The BLIiNG study—Breastfeeding length and intensity in gestational diabetes and metabolic effects in a subsequent pregnancy: A cohort study. Midwifery 2022, 107, 103262. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.P.; Stuebe, A.M.; Verbiest, S.B. The fourth trimester: A critical transition period with unmet maternal health needs. Am. J. Obstet. Gynecol. 2017, 217, 37–41. [Google Scholar] [CrossRef]
- Centre for Epidemiology and Evidence. New South Wales Mothers and Babies 2020; Ministry of Health: Sydney, Australia, 2021. [Google Scholar]
- Breastfeeding Report Card United States. 2022. Available online: https://www.cdc.gov/breastfeeding/data/reportcard.htm (accessed on 27 March 2023).
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.d.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- ADIPS. Consensus Guidelines for the Testing and Diagnosis of Hyperglycaemia in Pregnancy in Australia and New Zealand. Available online: https://www.adips.org/downloads/2014ADIPSGDMGuidelinesV18.11.2014.pdf (accessed on 4 April 2022).
- Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia. 2016. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/2033.0.55.001~2016~Main%20Features~IRSD~19 (accessed on 4 April 2022).
- Bandoli, G.; Palmsten, K.; Chambers, C.D.; Jelliffe-Pawlowski, L.L.; Baer, R.J.; Thompson, C.A. Revisiting the Table 2 fallacy: A motivating example examining preeclampsia and preterm birth. Paediatr. Perinat. Epidemiol. 2018, 32, 390–397. [Google Scholar] [CrossRef]
- Selen, D.J.; Thaweethai, T.; Schulte, C.C.; Hsu, S.; He, W.; James, K.; Kaimal, A.; Meigs, J.B.; Powe, C.E. Gestational Glucose Intolerance and Risk of Future Diabetes. Diabetes Care 2023, 46, 83–91. [Google Scholar] [CrossRef]
- Berezowsky, A.; Raban, O.; Aviram, A.; Zafrir-Danieli, H.; Krispin, E.; Hadar, E. Glucose tolerance test with a single abnormal value in pregnancy and the risk of type-2 diabetes mellitus. Arch. Gynecol. Obstet. 2022, 305, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.W.; Helmink, D. Gestational diabetes: The significance of persistent fasting hyperglycemia for the subsequent development of diabetes mellitus. J. Diabetes Complicat. 2006, 20, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Roeckner, J.T.; Sanchez-Ramos, L.; Jijon-Knupp, R.; Kaunitz, A.M. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 215, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Lu, J.; Zhang, L.; He, J.; Li, W.; Chen, N.; Wen, X.; Xiao, W.; Yuan, M.; Qiu, L.; et al. Single Fasting Plasma Glucose Versus 75-g Oral Glucose-Tolerance Test in Prediction of Adverse Perinatal Outcomes: A Cohort Study. eBioMedicine 2017, 16, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Stuebe, A.M.; Rich-Edwards, J.W. The reset hypothesis: Lactation and maternal metabolism. Am. J. Perinatol. 2009, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lai, M.; Piro, A.L.; Alexeeff, S.E.; Allalou, A.; Röst, H.L.; Dai, F.F.; Wheeler, M.B.; Gunderson, E.P. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: The SWIFT study. BMC Med. 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Gavine, A.; Shinwell, S.C.; Buchanan, P.; Farre, A.; Wade, A.; Lynn, F.; Marshall, J.; Cumming, S.E.; Dare, S.; McFadden, A. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst. Rev. 2022, 10, Cd001141. [Google Scholar] [PubMed]
- Marschner, S.; Chow, C.; Thiagalingam, A.; Simmons, D.; McClean, M.; Pasupathy, D.; Smith, B.J.; Flood, V.; Padmanabhan, S.; Melov, S.; et al. Effectiveness of a customised mobile phone text messaging intervention supported by data from activity monitors for improving lifestyle factors related to the risk of type 2 diabetes among women after gestational diabetes: Protocol for a multicentre randomised controlled trial (SMART MUMS with smart phones 2). BMJ Open 2021, 11, e054756. [Google Scholar]
- Melov, S.J.; Galas, N.; Swain, J.; Alahakoon, T.I.; Lee, V.; Cheung, N.W.; McGee, T.; Pasupathy, D.; McNab, J. Women’s experience of perinatal support in a high migrant Australian population during the COVID-19 pandemic: A mixed methods study. BMC Pregnancy Childbirth 2023, 23, 429. [Google Scholar] [CrossRef]
- Lachmann, E.H.; Fox, R.A.; Dennison, R.A.; Usher-Smith, J.A.; Meek, C.L.; Aiken, C.E. Barriers to completing oral glucose tolerance testing in women at risk of gestational diabetes. Diabet. Med. 2020, 37, 1482–1489. [Google Scholar] [CrossRef]
- Piper, S.; Parks, P.L. Use of an intensity ratio to describe breastfeeding exclusivity in a national sample. J. Hum. Lact. 2001, 17, 227–232. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [PubMed]
- Australian Institute of Health Welfare. 2010 Australian National Infant Feeding Survey: Indicator Results; Australian Institute of Health Welfare: Canberra, Australia, 2011. [Google Scholar]
- Westreich, D.; Greenland, S. The Table 2 Fallacy: Presenting and Interpreting Confounder and Modifier Coefficients. Am. J. Epidemiol. 2013, 177, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef]

| Maternal Characteristics | Duration of Breastfeeding Median Months (IQR) | p Value | High-Intensity Breastfeeding (n = 4074, 75.8%) | Low-Intensity Breastfeeding (n = 1300, 24.2%) | p Value |
|---|---|---|---|---|---|
| Maternal age | <0.001 | <0.001 | |||
| <25 | 6 (2–13) | 69.46% (232) | 30.54% (102) | ||
| 25–34 | 8 (4–15) | 74.30% (1772) | 25.70% (613) | ||
| 35–39 | 11 (6–18) | 77.96% (1765) | 22.04% (499) | ||
| >39 | 12 (6–18) | 78.01% (305) | 21.99% (86) | ||
| Ethnicity | <0.001 | <0.001 | |||
| South Asian | 12 (6–18) | 81.73% (1302) | 18.27% (291) | ||
| Southeast Asian | 10 (5–14) | 74.35% (661) | 25.65% (228) | ||
| White/European | 9 (4–14) | 73.09% (690) | 26.91% (254) | ||
| Middle Eastern | 7 (3–14) | 71.73% (789) | 28.27% (311) | ||
| Aboriginal and Torres Strait Islander | 4 (2–9) | 58.14% (50) | 41.86% (36) | ||
| Other | 8 (4–14) | 76.38% (582) | 23.62% (180) | ||
| Migrant status | <0.001 | <0.001 | |||
| <5 years | 12 (6–18) | 78.68% (1011) | 21.32% (274) | ||
| 5–10 years | 12 (6–18) | 78.57% (759) | 21.43% (207) | ||
| >10 years | 9 (4–15) | 75.64% (798) | 24.36% (267) | ||
| Australian-born | 7 (3–14) | 71.31% (1213) | 28.59% (488) | ||
| Socioeconomic status | <0.001 | <0.001 | |||
| Least advantaged Q1 | 8 (3–15) | 71.28% (1184) | 28.72% (477) | ||
| Q2 | 9 (4–14) | 75.83% (549) | 24.17% (175) | ||
| Q3 | 11 (6–18) | 77.56% (923) | 22.44% (267) | ||
| Q4 | 10 (5–17) | 80.09% (531) | 19.91% (132) | ||
| Most advantaged Q5 | 11 (5–16) | 78.06% (715) | 21.94% (201) | ||
| Parity | 0.006 | 0.024 | |||
| 1 | 10 (4–17) | 74.63% (2544) | 25.37% (865) | ||
| 2–3 | 8 (4–15) | 78.10% (1323) | 21.90% (371) | ||
| ≥4 | 8 (4–18) | 76.38% (207) | 23.62% (64) | ||
| Current pregnancy | |||||
| High risk of GDM * | 9 (4–16) | 0.859 | 74.61% (1740) | 25.39% (592) | 0.073 |
| Lower risk of GDM | 10 (4–16) | 76.73% (2334) | 23.27% (708) | ||
| Smoking | 4 (2–12) | <0.001 | 47.06% (48) | 52.94% (54) | <0.001 |
| No smoking | 10 (5–17) | 76.80% (2655) | 23.20% (802) | ||
| Comorbidities | |||||
| Polycystic ovary syndrome | 8 (4–16) | 0.23 | 73.11% (223) | 26.89% (82) | 0.26 |
| No polycystic ovary syndrome | 10 (4–16) | 75.97% (3850) | 24.03% (1218) | ||
| History of mental health issue | 7 (3–14) | <0.001 | 68.39% (541) | 31.61% (250) | <0.001 |
| No history of mental health issue | 10 (4–18) | 77.08% (3532) | 22.92% (1050) | ||
| History of hypertension | 8 (4–14) | 0.071 | 71.53% (206) | 28.47% (82) | 0.081 |
| No history of hypertension | 10 (4–16) | 76.05% (3867) | 23.95% (1218) | ||
| BMI kg/m2 | <0.001 | <0.001 | |||
| <18.5 | 8 (4–14) | 82.94% (141) | 17.06% (29) | ||
| 18.5–24.9 | 11 (5–17) | 77.77% (1907) | 22.23% (545) | ||
| 25.0–29.9 | 9 (4–17) | 77.04% (1258) | 22.96% (375) | ||
| ≥30.0 | 7 (3–15) | 68.63% (768) | 31.37% (351) | ||
| Previous pregnancy complications | |||||
| History of GDM | 9 (4–16) | 0.635 | 74.96% (518) | 25.04% (173) | 0.578 |
| No history of GDM | 9 (4–16) | 75.93% (3556) | 24.07% (1127) | ||
| Previous birth: caesarean section | 9 (4–18) | 0.851 | 72.94% (949) | 27.06% (352) | 0.006 |
| Previous birth: vaginal | 9 (4–16) | 76.72% (3125) | 23.28% (948) | ||
| Previous birth: preterm | 7 (4–14) | 0.003 | 67.89% (258) | 32.11% (122) | <0.001 |
| Previous birth term | 10 (4–17) | 76.41% (3816) | 23.59% (1178) |
| (a). High-Intensity Breastfeeding (HIBF)–Mostly or Exclusively Breastfeeding. | |||||||
| Outcome | Cohort | HIBF % (n) | LIBF % (n) | Unadjusted OR (95%CI) | p | Adjusted OR (95%CI) | p |
| Elevated Fasting OGTT | Total Cohort | 5.99% (243) | 8.02% (104) | 0.53 (0.38, 0.73) | <0.01 | 0.53 (0.38, 0.75) | <0.01 |
| High Risk | 8.93% (154) | 12.24% (72) | 0.56 (0.38, 0.83) | 0.01 | 0.55 (0.36, 0.83) | 0.01 | |
| Low Risk | 3.81% (89) | 4.52% (32) | 0.47 (0.27, 0.83) | 0.01 | 0.48 (0.27, 0.86) | 0.01 | |
| Elevated 1 h OGTT | Total Cohort | 10.72% (376) | 8.83% (100) | 1.14 (0.85, 1.52) | 0.37 | 1.20 (0.89, 1.63) | 0.23 |
| High Risk | 16.43% (236) | 14.57% (73) | 1.04 (0.73, 1.47) | 0.84 | 1.05 (0.72, 1.52) | 0.81 | |
| Low Risk | 6.75% (140) | 4.28% (27) | 1.52 (0.90, 2.59) | 0.12 | 1.58 (0.92, 2.71) | 0.09 | |
| Elevated 2 h OGTT | Total Cohort | 10.38% (420) | 10.78% (139) | 0.96 (0.73, 1.26) | 0.77 | 1.01 (0.76, 1.33) | 0.97 |
| High Risk | 16.07% (276) | 15.92% (93) | 0.91 (0.66, 1.26) | 0.57 | 0.92 (0.65, 1.29) | 0.62 | |
| Low Risk | 6.18% (144) | 6.52% (46) | 1.18 (0.73, 1.93) | 0.50 | 1.23 (0.75, 2.01) | 0.42 | |
| Diagnosed GDM | Total Cohort | 18.09% (737) | 18.77% (244) | 0.87 (0.73, 1.03) | 0.11 | 0.91 (0.75, 1.10) | 0.34 |
| High Risk | 26.72% (465) | 27.87% (165) | 0.86 (0.68, 1.07) | 0.18 | 0.84 (0.65, 1.08) | 0.18 | |
| Low Risk | 11.65% (272) | 11.16% (79) | 0.95 (0.71, 1.27) | 0.75 | 1.02 (0.75, 1.37) | 0.92 | |
| (b). Breastfeeding duration as a continuous variable and elevated glucose. | |||||||
| Outcome | Cohort | Normal Glucose BF Months (IQR) | Elevated Glucose BF months (IQR) | Unadjusted OR (95%CI) | p | Adjusted OR (95%CI) | p |
| Fasting OGTT | Total Cohort | 9 (4–16) | 11 (4–18) | 1.03 (1.01, 1.04) | <0.01 | 1.02 (1.00, 1.04) | 0.04 |
| High Risk | 9 (4–16) | 9 (4–18) | 1.02 (1.00, 1.04) | 0.02 | 1.02 (0.99, 1.04) | 0.15 | |
| Low Risk | 9 (4–16) | 12 (6–19) | 1.04 (1.01, 1.07) | 0.01 | 1.03 (0.99, 1.06) | 0.06 | |
| 1 h OGTT | Total Cohort | 9 (4–16) | 12 (6–18) | 1.02 (1.00, 1.03) | 0.01 | 1.01 (0.99, 1.02) | 0.34 |
| High Risk | 9 (4–15) | 11.5 (6–18) | 1.02 (1.00, 1.04) | 0.02 | 1.05 (0.72, 1.52) | 0.81 | |
| Low Risk | 9 (4–16) | 12 (6–18) | 1.01 (0.99, 1.04) | 0.23 | 1.00 (0.98, 1.03) | 0.84 | |
| 2 h OGTT | Total Cohort | 9 (4–16) | 11 (5–18) | 1.01 (1.00, 1.02) | 0.12 | 1.00 (0.99, 1.02) | 0.86 |
| High Risk | 9 (4–15) | 11 (5–18) | 1.02 (1.00, 1.04) | 0.01 | 1.01 (0.99, 1.03) | 0.62 | |
| Low Risk | 9 (4–16) | 11 (5–18) | 0.99 (0.97, 1.02) | 0.50 | 0.98 (0.96, 1.01) | 0.14 | |
| GDM | Total Cohort | 9 (4–16) | 11 (5–18) | 1.02 (1.01, 1.02) | <0.01 | 1.01 (0.99, 1.02) | 0.06 |
| High Risk | 9 (4–16) | 11 (5–18) | 1.02 (1.00, 1.03) | 0.01 | 1.01 (0.99, 1.02) | 0.15 | |
| Low Risk | 9 (4–16) | 12 (5–18) | 1.02 (1.00, 1.03) | 0.03 | 1.01 (0.99, 1.02) | 0.21 | |
| Characteristic | High-Intensity Breastfeeding | Breastfeeding Duration > 6 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95%CI) | p | aOR (95%CI) | p | Unadjusted OR (95%CI) | p | aOR (95%CI) | p | |
| Maternal age | ||||||||
| <25 | 0.79 (0.61, 1.01) | ns | 0.95 (0.73, 1.23) | ns | 0.62 (0.49, 0.78) | <0.01 | 0.74 (0.58, 0.94) | 0.01 |
| 25–34 | Ref | - | - | - | Ref | - | - | - |
| 35–39 | 1.22 (1.07, 1.40) | 0.01 | 1.10 (0.96, 1.27) | ns | 1.40 (1.24, 1.58) | <0.01 | 1.25 (1.10, 1.42) | <0.01 |
| 40+ | 1.23 (0.95, 1.59) | ns | 1.17 (0.89, 1.53) | ns | 1.53 (1.22, 1.93) | <0.01 | 1.52 (1.20, 1.94) | <0.01 |
| Ethnicity | ||||||||
| South Asian | 1.65 (1.36, 2.00) | <0.01 | 1.65 (1.36, 2.00) | <0.01 | 1.81 (1.52, 2.15) | <0.01 | 1.81 (1.52, 2.15) | <0.01 |
| South-East Asian | 1.07 (0.87, 1.31) | ns | 1.07 (0.87, 1.31) | ns | 1.27 (1.04, 1.54) | 0.02 | 1.27 (1.04, 1.54) | 0.02 |
| White/European | Ref | - | - | - | Ref | - | - | - |
| Middle Eastern | 0.93 (0.77, 1.13) | ns | 0.93 (0.77, 1.13) | ns | 0.79 (0.66, 0.94) | 0.01 | 0.79 (0.66, 0.94) | 0.01 |
| Aboriginal/Torres Strait Islander | 0.51 (0.33, 0.80) | 0.01 | 0.51 (0.33, 0.80) | 0.01 | 0.31 (0.19, 0.50) | <0.01 | 0.31 (0.19, 0.50) | <0.01 |
| Other | 1.19 (0.95, 1.48) | ns | 1.19 (0.95, 1.48) | ns | 0.97 (0.79, 1.18) | ns | 0.97 (0.79, 1.18) | ns |
| Migrant status | ||||||||
| <5 years | 1.48 (1.25, 1.76) | <0.01 | 1.20 (0.97, 1.48) | ns | 1.94 (1.66, 2.26) | <0.01 | 1.60 (1.32, 1.94) | <0.01 |
| 5–10 years | 1.48 (1.22, 1.78) | <0.01 | 1.18 (0.94, 1.47) | ns | 1.95 (1.65, 2.31) | <0.01 | 1.56 (1.27, 1.91) | <0.01 |
| >10 years | 1.25 (1.05, 1.49) | 0.01 | 1.10 (0.90, 1.35) | ns | 1.51 (1.29, 1.77) | <0.01 | 1.32 (1.09, 1.59) | 0.01 |
| Australian-born | Ref | - | - | - | Ref | - | - | - |
| Previous complications | ||||||||
| History of GDM | 0.95 (0.79, 1.14) | ns | 0.92 (0.76, 1.11) | ns | 1.05 (0.88, 1.24) | ns | 0.97 (0.82, 1.15) | ns |
| History of hypertension | 0.79 (0.61, 1.03) | ns | 0.88 (0.68, 1.15) | ns | 0.90 (0.70, 1.14) | ns | 1.03 (0.80, 1.33) | ns |
| Previous birth caesarean section | 0.82 (0.71, 0.94) | 0.01 | 0.78 (0.67, 0.91) | <0.01 | 0.93 (0.82, 1.06) | ns | 0.85 (0.74, 0.97) | 0.02 |
| Previous birth preterm | 0.65 (0.52, 0.82) | <0.01 | 0.61 (0.48, 0.77) | <0.01 | 0.73 (0.59, 0.91) | 0.01 | 0.76 (0.60, 0.95) | 0.02 |
| Current pregnancy | ||||||||
| Smoking | 0.27 (0.18, 0.40) | <0.01 | 0.31 (0.21, 0.47) | <0.01 | 0.33 (0.22, 0.49) | <0.01 | 0.44 (0.29, 0.67) | <0.01 |
| BMI kg/m2 | ||||||||
| <18.5 | 1.39 (0.92, 2.10) | ns | 1.53 (1.01, 2.32) | ns | 0.87 (0.63, 1.21) | ns | 0.96 (0.69, 1.33) | ns |
| 18.5–24.9 | Ref | - | - | - | Ref | - | - | - |
| 25.0–29.9 | 0.96 (0.83, 1.11) | ns | 0.89 (0.77, 1.04) | ns | 0.87 (0.76, 0.99) | 0.04 | 0.86 (0.75, 0.99) | 0.04 |
| ≥30.0 | 0.63 (0.53, 0.73) | <0.01 | 0.62 (0.52, 0.73) | <0.01 | 0.59 (0.51, 0.68) | <0.01 | 0.69 (0.57, 0.78) | <0.01 |
| History of mental health issue | 0.64 (0.55, 0.76) | <0.01 | 0.69 (0.58, 0.83) | <0.01 | 0.67 (0.57, 0.78) | <0.01 | 0.78 (0.65, 0.91) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melov, S.J.; Elhindi, J.; White, L.; McNab, J.; Lee, V.W.; Donnolley, K.; Alahakoon, T.I.; Padmanabhan, S.; Cheung, N.W.; Pasupathy, D. Previous High-Intensity Breastfeeding Lowers the Risk of an Abnormal Fasting Glucose in a Subsequent Pregnancy Oral Glucose Tolerance Test. Nutrients 2024, 16, 28. https://doi.org/10.3390/nu16010028
Melov SJ, Elhindi J, White L, McNab J, Lee VW, Donnolley K, Alahakoon TI, Padmanabhan S, Cheung NW, Pasupathy D. Previous High-Intensity Breastfeeding Lowers the Risk of an Abnormal Fasting Glucose in a Subsequent Pregnancy Oral Glucose Tolerance Test. Nutrients. 2024; 16(1):28. https://doi.org/10.3390/nu16010028
Chicago/Turabian StyleMelov, Sarah J., James Elhindi, Lisa White, Justin McNab, Vincent W. Lee, Kelly Donnolley, Thushari I. Alahakoon, Suja Padmanabhan, N. Wah Cheung, and Dharmintra Pasupathy. 2024. "Previous High-Intensity Breastfeeding Lowers the Risk of an Abnormal Fasting Glucose in a Subsequent Pregnancy Oral Glucose Tolerance Test" Nutrients 16, no. 1: 28. https://doi.org/10.3390/nu16010028