Chemical Constituents, Biological Activities, and Proposed Biosynthetic Pathways of Steroidal Saponins from Healthy Nutritious Vegetable—Allium
Abstract
:1. Introduction
2. Chemical Structures of Allium Steroidal Saponins
2.1. Furostane Saponins/Sapogenins
2.2. Spirostane Saponins/Sapogenins
2.3. Cholestane Saponins/Sapogenins
2.4. Other Types of Steroidal Saponins/Sapogenins
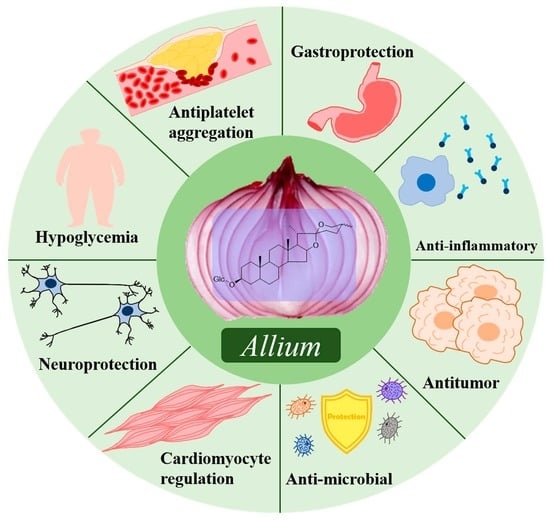
3. Biological Activities of Allium Steroidal Saponins
3.1. Hypoglycemic Effect
3.2. Antiplatelet Aggregation Effect
3.3. Gastroprotective Effect
3.4. Immune Adjuvant Effect
3.5. Anti-Inflammatory Effect
3.6. Cytotoxicity and Antitumor Effects
3.7. Antimicrobial Effect
3.8. Enzyme Activity Inhibition Effect
3.9. Antispasmodic Effect
3.10. Cardiomyocyte Regulation Effect
3.11. Nerve Cell Protection Effect
3.12. Hemolysis Effect
4. Proposed Biosynthetic Pathways of Allium Steroidal Saponins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omar, S.H.; Al-Wabel, N.A. Organosulfur Compounds and Possible Mechanism of Garlic in Cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Hayashi, N.; Fuwa, T. A Furostanol Glycoside from Garlic, Bulbs of Allium sativum L. Chem. Pharm. Bull. 1988, 36, 3659–3663. [Google Scholar] [CrossRef]
- Morita, T.; Ushiroguchi, T.; Hayashi, N.; Matsuura, H.; Itakura, Y.; Fuwa, T. Steroidal Saponins from Elephant Garlic, Bulbs of Allium ampeloprasum L. Chem. Pharm. Bull. 1988, 36, 3480–3486. [Google Scholar] [CrossRef]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Fuwa, T. Further Studies on Steroidal Glycosides from Bulbs, Roots and Leaves of Allium sativum L. Chem. Pharm. Bull. 1989, 37, 2741–2743. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kawashima, K.; Kanmoto, T.; Sashida, Y. Steroidal Glycosides from Allium albopilosum and A. ostrowskianum. Phytochemistry 1993, 34, 799–805. [Google Scholar] [CrossRef]
- Mimaki, Y.; Nikaido, T.; Matsumoto, K.; Sashida, Y.; Ohmoto, T. New Steroidal Saponins from the Bulbs of Allium giganteum Exhibiting Potent Inhibition of CAMP Phosphodiesterase Activity. Chem. Pharm. Bull. 1994, 42, 710–714. [Google Scholar] [CrossRef]
- Kawashima, K.; Mimaki, Y.; Sashida, Y. Steroidal Saponins from the Bulbs of Allium schubertii. Phytochemistry 1993, 32, 1267–1272. [Google Scholar] [CrossRef]
- Peng, J.; Yao, X.; Okada, Y.; Okuyama, T. Further Studies on New Furostanol Saponins from the Bulbs of Allium macrostemon. Chem. Pharm. Bull. 1994, 42, 2180–2182. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Fukasawa, T.; Sashida, Y. Steroidal Saponins from the Bulbs of Allium karataviense. Chem. Pharm. Bull. 1999, 47, 738–743. [Google Scholar] [CrossRef]
- Barile, E.; Zolfaghari, B.; Sajjadi, S.E.; Lanzotti, V. Saponins of Allium elburzense. J. Nat. Prod. 2004, 67, 2037–2042. [Google Scholar] [CrossRef]
- Ikeda, T.; Tsumagari, H.; Okawa, M.; Nohara, T. Pregnane- and Furostane-Type Oligoglycosides from the Seeds of Allium tuberosum. Chem. Pharm. Bull. 2004, 52, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Barile, E.; Capasso, R.; Izzo, A.A.; Lanzotti, V.; Sajjadi, S.E.; Zolfaghari, B. Structure-Activity Relationships for Saponins from Allium hirtifolium and Allium elburzense and Their Antispasmodic Activity. Planta Med. 2005, 71, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Yiran, Y.; Hua, Y.; Jianghong, Y.; Zhiheng, S.; Yu, Z.; Xueqing, F.; Xuwen, L.; Yongri, J. Chemical Constituents of New Steroidal Saponins from Allium chinense G. Don. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2021, 42, 1742–1753. [Google Scholar] [CrossRef]
- Barile, E.; Bonanomi, G.; Antignani, V.; Zolfaghari, B.; Sajjadi, S.E.; Scala, F.; Lanzotti, V. Saponins from Allium minutiflorum with Antifungal Activity. Phytochemistry 2007, 68, 596–603. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Wang, N.; Yang, M.; Wang, Z.; Wang, X.; Yao, X. New Furostanol Saponins from the Bulbs of Allium macrostemon Bunge and Their Cytotoxic Activity. Pharmazie 2007, 62, 544–548. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, X.; Xiang, L.; Huang, Y.; Wang, Z.; He, X. Furostanol Saponins from Chinese Onion Induce G2/M Cell-Cycle Arrest and Apoptosis through Mitochondria-Mediate Pathway in HepG2 Cells. Steroids 2019, 148, 11–18. [Google Scholar] [CrossRef]
- Chen, H.-F.; Wang, N.-L.; Sun, H.-L.; Yang, B.-F.; Yao, X.-S. Novel Furostanol Saponins from the Bulbs of Allium macrostemon B. and Their Bioactivity on [Ca2+]i Increase Induced by KCl. J. Asian Nat. Prod. Res. 2006, 8, 21–28. [Google Scholar] [CrossRef]
- Chen, H.; Ou, W.; Wang, G.; Wang, N.; Zhang, L.; Yao, X. New Steroidal Glycosides Isolated as CD40L Inhibitors of Activated Platelets. Molecules 2010, 15, 4589–4598. [Google Scholar] [CrossRef]
- Maisashvili, M.R.; Kuchukhidze, D.K.; Kikoladze, V.S.; Gvazava, L.N. Steroidal Glycosides of Gitogenin from Allium rotundum. Chem. Nat. Compd. 2012, 48, 86–90. [Google Scholar] [CrossRef]
- Lanzotti, V.; Barile, E.; Antignani, V.; Bonanomi, G.; Scala, F. Antifungal Saponins from Bulbs of Garlic, Allium sativum L. Var. Voghiera. Phytochemistry 2012, 78, 126–134. [Google Scholar] [CrossRef]
- Sadeghi, M.; Zolfaghari, B.; Senatore, M.; Lanzotti, V. Spirostane, Furostane and Cholestane Saponins from Persian Leek with Antifungal Activity. Food Chem. 2013, 141, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Mskhiladze, L.; Chincharadze, D.; Mshvildadze, V.; Pichette, A.; Frederich, M.; Ollivier, E.; Elias, R. Steroidal Glycosides from the Flowers of Allium leucanthum. Chem. Nat. Compd. 2015, 51, 900–904. [Google Scholar] [CrossRef]
- Kang, L.-P.; Liu, Z.-J.; Zhang, L.; Tan, D.-W.; Zhao, Y.; Zhao, Y.; Chen, H.-B.; Ma, B.-P. New Furostanol Saponins from Allium ascalonicum L. Magn. Reson. Chem. 2007, 45, 725–733. [Google Scholar] [CrossRef]
- Timité, G.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Mirjolet, J.-F.; Duchamp, O.; Lacaille-Dubois, M.-A. Structure and Cytotoxicity of Steroidal Glycosides from Allium schoenoprasum. Phytochemistry 2013, 88, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Ori, K.; Takayama, H.; Sakagami, H.; Mimaki, Y. Karataviosides G–K, Five New Bisdesmosidic Steroidal Glycosides from the Bulbs of Allium karataviense. Steroids 2015, 93, 96–104. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, W.S.; Park, K.J.; Choi, S.U.; Lee, K.R. Allimacrosides A–E, New Steroidal Glycosides from Allium macrostemon Bunge. Steroids 2017, 118, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Mao, S.; Lao, A.; Chen, Z.; Ho, C.-T. Four New Steroidal Saponins from the Seeds of Allium tuberosum. J. Agric. Food Chem. 2001, 49, 1475–1478. [Google Scholar] [CrossRef]
- Sang, S.; Lao, A.; Wang, H.; Chen, Z. Furostanol Saponins from Allium tuberosum. Phytochemistry 1999, 52, 1611–1615. [Google Scholar] [CrossRef]
- Sang, S.; Mao, S.; Lao, A.; Chen, Z.; Ho, C.-T. New Steroid Saponins from the Seeds of Allium tuberosum L. Food Chem. 2003, 83, 499–506. [Google Scholar] [CrossRef]
- Peng, J.; Yao, X.; Kobayashi, H.; Ma, C. Novel Furostanol Glycosides from Allium macrostemon. Planta Med. 1995, 61, 58–61. [Google Scholar] [CrossRef]
- Chen, H.-F.; Wang, G.-H.; Luo, Q.; Wang, N.-L.; Yao, X.-S. Two New Steroidal Saponins from Allium macrostemon Bunge and Their Cytotoxity on Different Cancer Cell Lines. Molecules 2009, 14, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ushiroguchi, T.; Itakura, Y.; Fuwa, T. A Furostanol Glycoside from Allium chinense G. DON. Chem. Pharm. Bull. 1989, 37, 1390–1391. [Google Scholar] [CrossRef]
- Peng, J.-P.; Yao, X.-S.; Tezuka, Y.; Kikuchi, T. Furostanol Glycosides from Bulbs of Allium chinense. Phytochemistry 1996, 41, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yao, X.; Tezuka, Y.; Kikuchi, T.; Narui, T. New Furostanol Glycosides, Chinenoside IV and V, from Allium chinense. Planta Med. 1996, 62, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Mimaki, Y.; Kuroda, M.; Fukasawa, T.; Sashida, Y. Steroidal Glycosides from the Bulbs of Allium jesdianum. J. Nat. Prod. 1999, 62, 194–197. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Di Rosa, M.; Ianaro, A. Cytotoxic Saponins from Bulbs of Allium porrum L. J. Agric. Food Chem. 2000, 48, 3455–3462. [Google Scholar] [CrossRef]
- Harmatha, J.; Buděšínský, M.; Zídek, Z.; Kmoníčková, E. Spirostanol Saponins from Flowers of Allium porrum and Related Compounds Indicating Cytotoxic Activity and Affecting Nitric Oxide Production Inhibitory Effect in Peritoneal Macrophages. Molecules 2021, 26, 6533. [Google Scholar] [CrossRef]
- Tolkacheva, N.V.; Shashkov, A.S.; Chirva, V.Y. Steroidal Glycosides from Allium cyrillii Bulbs. Chem. Nat. Compd. 2012, 48, 272–275. [Google Scholar] [CrossRef]
- Kawashima, K.; Mimaki, Y.; Sashida, Y. Steroidal Saponins from Allium giganteum and A. aflatunense. Phytochemistry 1991, 30, 3063–3067. [Google Scholar] [CrossRef]
- Inoue, T.; Mimaki, Y.; Sashida, Y.; Nishino, A.; Satomi, Y.; Nishino, H. Steroidal Glycosides from Allium macleanii and A. senescens, and Their Inhibitory Activity on Tumour Promoter-Induced Phospholipid Metabolism of Hela Cells. Phytochemistry 1995, 40, 521–525. [Google Scholar] [CrossRef]
- Sata, N.; Matsunaga, S.; Fusetani, N.; Nishikawa, H.; Takamura, S.; Saito, T. New Antifungal and Cytotoxic Steroidal Saponins from the Bulbs of an Elephant Garlic Mutant. Biosci. Biotechnol. Biochem. 1998, 62, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Mskhiladze, L.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Kuchukhidze, J.; Elias, R.; Pichette, A. Cytotoxic Steroidal Saponins from the Flowers of Allium leucanthum. Molecules 2008, 13, 2925–2934. [Google Scholar] [CrossRef] [PubMed]
- Jabrane, A.; Ben Jannet, H.; Miyamoto, T.; Mirjolet, J.-F.; Duchamp, O.; Harzallah-Skhiri, F.; Lacaille-Dubois, M.-A. Spirostane and Cholestane Glycosides from the Bulbs of Allium nigrum L. Food Chem. 2011, 125, 447–455. [Google Scholar] [CrossRef]
- Mostafa, A.; Sudisha, J.; El-Sayed, M.; Ito, S.; Ikeda, T.; Yamauchi, N.; Shigyo, M. Aginoside Saponin, a Potent Antifungal Compound, and Secondary Metabolite Analyses from Allium nigrum L. Phytochem. Lett. 2013, 6, 274–280. [Google Scholar] [CrossRef]
- Sashida, Y.; Kawashima, K.; Mimaki, Y. Novel Polyhydroxylated Steroidal Saponins from Allium giganteum. Chem. Pharm. Bull. 1991, 39, 698–703. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Kameyama, A.; Sashida, Y.; Nikaido, T. Steroidal Saponins from Allium chinense and Their Inhibitory Activities on Cyclic AMP Phosphodiesterase and Na+K+ ATPase. Phytochemistry 1995, 40, 1071–1076. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Wang, N.; Zhang, Y.; Cai, G. Macrostemonoside A Promotes Visfatin Expression in 3T3-L1 Cells. Biol. Pharm. Bull. 2007, 30, 279–283. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S.; De Feo, V.; Carnuccio, R.; D’Acquisto, F. Porrigenins A and B, Novel Cytotoxic and Antiproliferative Sapogenins Isolated from Allium porrum. J. Nat. Prod. 1997, 60, 1003–1007. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, N.-L.; Yao, X.-S.; Kitanaka, S. Steroidal Saponins from the Bulbs of Allium chinense. Stud. Plant. Sci. 1999, 6, 212–219. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S. Spirostanol Saponins of Allium porrum L. Phytochemistry 1999, 51, 1077–1082. [Google Scholar] [CrossRef]
- Sang, S.; Lao, A.; Wang, H.; Chen, Z. Two New Spirostanol Saponins from Allium tuberosum. J. Nat. Prod. 1999, 62, 1028–1029. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsumagari, H.; Nohara, T. Steroidal Oligoglycosides from the Seeds of Allium tuberosum. Chem. Pharm. Bull. 2000, 48, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Zou, M.; Xia, Z.; Lao, A.; Chen, Z.; Ho, C.-T. New Spirostanol Saponins from Chinese Chives (Allium tuberosum). J. Agric. Food Chem. 2001, 49, 4780–4783. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-M.; Yu, D.-Q.; Cong, P.-Z. A Steroidal Saponin from the Seeds of Allium tuberosum. Phytochemistry 2001, 57, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.-S.; Cai, L.; Li, Y.; Wang, J.-P.; Xiao, H.; Ding, Z.-T. Spirostanol Steroids from the Roots of Allium tuberosum. Steroids 2015, 100, 1–4. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Barile, E.; Capasso, R.; Izzo, A.A.; Sajjadi, S.E.; Lanzotti, V. The Sapogenin Atroviolacegenin and Its Diglycoside Atroviolaceoside from Allium atroviolaceum. J. Nat. Prod. 2006, 69, 191–195. [Google Scholar] [CrossRef]
- Adão, C.R.; da Silva, B.P.; Parente, J.P. A New Steroidal Saponin from Allium ampeloprasum Var. porrum with Antiinflammatory and Gastroprotective Effects. Phytochem. Lett. 2011, 4, 306–310. [Google Scholar] [CrossRef]
- Hu, G.; Mao, R.; Ma, Z. A New Steroidal Saponin from the Seeds of Allium tuberosum. Food Chem. 2009, 113, 1066–1068. [Google Scholar] [CrossRef]
- Adão, C.R.; da Silva, B.P.; Parente, J.P. A New Steroidal Saponin with Antiinflammatory and Antiulcerogenic Properties from the Bulbs of Allium ampeloprasum Var. porrum. Fitoterapia 2011, 82, 1175–1180. [Google Scholar] [CrossRef]
- Hu, G.; Lu, Y.; Yu, W.; Ding, Q.; Yang, Q.; Zhou, J.; Ma, Z. A Steroidal Saponin from the Seeds of Allium tuberosum. Chem. Nat. Compd. 2014, 49, 1082–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, L.; Huang, W.; He, X. Spirostanol Saponins from Chinese Onion (Allium chinense) Exert Pronounced Anti-Inflammatory and Anti-Proliferative Activities. J. Funct. Foods 2016, 25, 208–219. [Google Scholar] [CrossRef]
- Mirsalikhova, N.M.; Kravets, S.S.; Sokolova, S.F.; Abubakirov, N.K. Inhibition of Highly Purified Porcine Kidney Na,K-ATPase by Steroid Glycosides of the Spirostan and Furostan Series and a Study of Structure-Activity Relationships. Chem. Nat. Compd. 1993, 29, 490–497. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Mahmoud, H.Y.A.H.; El-Sayed, M.; Tanaka, S.; Tran, L.S. Isolation and Characterization of Cepa2, a Natural Alliospiroside A, from Shallot (Allium cepa L. Aggregatum Group) with Anticancer Activity. Plant Physiol. Biochem. 2017, 116, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Adão, C.R.; Pereira da Silva, B.; Tinoco, L.W.; Parente, J.P. Haemolytic Activity and Immunological Adjuvant Effect of a New Steroidal Saponin from Allium ampeloprasum Var. porrum. Chem. Biodivers 2012, 9, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Zolfaghari, B.; Troiano, R.; Lanzotti, V. 3-Keto Umbilicagenin A and B, New Sapogenins from Allium umbilicatum Boiss. Fitoterapia 2015, 102, 198–202. [Google Scholar] [CrossRef]
- Vollerner, Y.S.; Kravits, S.D.; Shashkov, A.S.; Gorovits, M.B.; Abubakirov, N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. Structure of Anzurogenin A from Allium suvorovii and A. stipitatum. Chem. Nat. Compd. 2004, 24, 58–62. [Google Scholar] [CrossRef]
- Vollerner, Y.S.; Kravets, S.D.; Shashkov, A.S.; Gorovits, M.B.; Abubakirov, N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. XXVI. Structure of Anzurogenin and Anzuroside from the Collective Fruits of Allium suvorovii and Allium stipitatum. Chem. Nat. Compd. 1989, 25, 431–435. [Google Scholar] [CrossRef]
- Baba, M.; Matsuda, M.O.; Kishi, N.; Okada, Y.; Shibata, S.; Peng, J.; Yao, S.-S.; Nishino, H.; Okuyama, T. Saponins Isolated from Allium Chinense G. DON and Antitumor-Promoting Activities of Isoliquiritigenin and Laxogenin from the Same Drug. Biol. Pharm. Bull. 2000, 23, 660–662. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fattorusso, E.; Lanzotti, V.; Magno, S.; Carnuccio, R.; D’Acquisto, F. 12-Keto-Porrigenin and the Unique 2,3-Seco-Porrigenin, New Antiproliferative Sapogenins from Allium porrum. Tetrahedron 1997, 53, 3401–3406. [Google Scholar] [CrossRef]
- Gvazava, L.N.; Skhirtladze, A.V. Steroidal Saponin from Allium porrum. Chem. Nat. Compd. 2017, 53, 1093–1095. [Google Scholar] [CrossRef]
- Fattorusso, E.; Lanzotti, V.; Magno, S.; Taglialatela-Scafati, O. Sapogenins of Allium porrum L. J. Agric. Food Chem. 1998, 46, 4904–4908. [Google Scholar] [CrossRef]
- Gvazava, L.N.; Skhirtladze, A.V. Steroidal Glycoside from Allium porrum. Chem. Nat. Compd. 2018, 54, 487–489. [Google Scholar] [CrossRef]
- Lai, W.; Yang, Y.B.; Li, X.; Sun, L.N.; Wu, Z.J.; Chen, W.S. New Steroidal Sapogenins from the Acid Hydrolysis Product of the Whole Glycoside Mixture of Welsh Onion Seeds. Chin. Chem. Lett. 2012, 23, 193–196. [Google Scholar] [CrossRef]
- Vollerner, Y.S.; Kravets, S.D.; Shashkov, A.S.; Gorovits, M.B.; Abubakirov, N.K. Steroids of the Spirostan and Furostan Series from Plants of the Genus Allium. XXV. Structure of Anzurogenin B from Allium Suvorovii and Allium Stipitatum. Chem. Nat. Compd. 1988, 24, 183–186. [Google Scholar] [CrossRef]













| No. | Common Name | Structure Name | Species | Parts | References |
|---|---|---|---|---|---|
| 1 | proto-eruboside-B | 26-O-β-glucopyranosyl 22-hydroxy-25(R)-5α-furostane-3β,6β-26-triol 3-O-β-glucopyranosyl-(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. sativum L | bulbs | [2] |
| 2 | ampeloside Bf1 | (25R)-26-O-β-glucopyranosyl-22-hydroxy-5α-furostane-2α,3β,6β,26-tetraol-3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| 3 | ampeloside Bf2 | (25R)-26-O-β-glucopyranosyl-22-hydroxy-5α-furostane-2α,3β,6β,26-tetraol-3-O-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| 4 | sativoside-B1 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furostane-3β,6β,26-triol 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. sativum L | bulbs | [4] |
| 5 | proto-desgalactotigonin | A. sativum L | bulbs and roots | [4] | |
| 6 | sativoside-R1 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furostane-3β,26-diol 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyde | A. sativum L | roots | [4] |
| 7 | 22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,6β,22ξ,26-pentol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| A. ostrowskianum | bulbs | [5] | |||
| A. giganteum | bulbs | [6] | |||
| 8 | 26-O-β-D-glucopyranosyl-(25S)-5α-furostane-2α,3β,6β,22ξ,26-pentaol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| A. ostrowskianum | bulbs | [5] | |||
| 9 | 26-O-β-D-glucopyranosyl-(25R)-5α-furostan-2α,3β,6β,22ξ,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 10 | 26-O-β-D-glucopyranosyl-(25S)-5α-furostan-2α,3β,6β,22ξ,26-pentol 3-O-β-D-glucopyranosyl-(l→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 11 | macrostemonoside J | 26-O-β-D-glucopyranosyl 2β,3β,22,26-tetrahydroxy-25(R)-5β-furostan 3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [8] |
| 12 | 3-O-benzoyl-22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 13 | 3-O-acetyl-22-O-methyl-26-O-β-D-glucopyranosyl-(25R)-5α-furostane-2α,3β,5α,6β,22ξ,26-hexol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 14 | (25R)-26-O-β-D-glucopyranosyl-22-O-methyl-5α-furostane-2α,3β,5,6β,22ξ-pentol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 15 | elburzensosides A1 | furost-2α,3β,5α,6β,22α-pentol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 16 | elburzensosides A2 | furost-2α,3β,5α,6β,22β-pentol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 17 | elburzensosides B1 | furost-2α,3β,5α,6β,22α-pentol 3-O-[β-D-glucopyranosyl-(1→4)-O-β-D-glucopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 18 | elburzensosides B2 | furost-2α,3β,5α,6β,22β-pentol 3-O-[β-D-glucopyranosyl-(1→4)-O-β-D-glucopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 19 | elburzensosides C1 | furost-2α,3β,5α,22α-tetrol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 20 | elburzensosides C2 | furost-2α,3β,5α,22β-tetrol 3-O-β-D-glucopyranosyl 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 21 | elburzensosides D1 | furost-2α,3β,5α,22α-tetrol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 22 | elburzensosides D2 | furost-2α,3β,5α,22β-tetrol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. elburzense | bulbs | [10] |
| 23 | 26-O-β-D-glucopyranosyl-(25R)-3β,22ξ,26-trihydroxyl-5α-furostane 3-O-β-chacotrioside | A. tuberosum Rottler | seeds | [11] | |
| 24 | 26-O-β-D-glucopyranosyl-(25S)-3β,5β,6α,22ξ,26-pentahydroxyl-5β-furostane 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum Rottler | seeds | [11] | |
| 25 | hirtifolioside A1 | furost-2α,3β,22α-triol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 26 | hirtifolioside A2 | furost-2α,3β,22β-triol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 27 | hirtifolioside C1 | (25R)-5α-furostane-2α,3β,22α,26-tetraol-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| A. chinense G. Don | bulbs | [13] | |||
| 28 | hirtifolioside C2 | furost-2α,3β,22β-triol 26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 29 | minutoside A | (25R)-furost-2α,3β,6β,22α,26-pentaol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 30 | minutoside C | (25R)-furost-2α,3β,5α,6β,22α,26-esaol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl] 26-O-β-D-glucopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 31 | macrostemonoside P | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furostane-1β,3β, 26-triol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 32 | macrostemonoside Q | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-1α,2β,3β, 26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 33 | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5β-furostane-3β, 26-diol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] | |
| 34 | macrostemonoside R | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-furostane-2α,3β,26-triol-3-O-β-D-glucopyranosyl (1→2)-[β-D-glucopyranosyl (1→3)]-β-D-glucopyranosyl (1→4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| A. chinense G. Don | bulbs | [16] | |||
| 35 | macrostemonoside B | A. macrostemon Bunge | bulbs | [15] | |
| 36 | macrostemonoside M | (25R)-22-hydroxy-5β-furostane-1β,2β,3β,6α-tetraol-26-O-β-D-glucopyranoside | A. macrostemon Bunge | bulbs | [17] |
| 37 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12β,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2) [β-D-glucopyranosyl (1 →3)]-β-D-glucopyranosyl (1 →4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 38 | (25R)-26-O-β-D-glucopyranosyl-5α-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2) [β-D-glucopyranosyl (1→3)]-β-D-glucopyranosyl (1 →4) -β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 39 | (25R)-26-O-β-D-glucopyranosyl-5β-furostane-3β,12α,22,26-tetraol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [18] | |
| 40 | 26-O-β-D-glucopyranosyl-(25R)-5α-furostan-2α,3β,22α,26-tetraol 3-O-β-D-glucopyranosyl-(1→2)[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. rotundum | inflorescences and flower stalks | [19] | |
| 41 | voghieroside A1 | furosta-2α,3β,5α,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 42 | voghieroside A2 | furosta-2α,3β,5α,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 43 | voghieroside B1 | furosta-2α,3β,5α,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 44 | voghieroside B2 | furosta-2α,3β,5α,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 45 | voghieroside C1 | furosta-2α,3β,6β,22α,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl -26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 46 | voghieroside C2 | furosta-2α,3β,6β,22β,26-pentol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl -26-O-β-D-glucopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 47 | voghieroside D1 | furosta-2α,3β,22α,26-tetrol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 48 | voghieroside D2 | furosta-2α,3β,22β,26-tetrol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 49 | voghieroside E1 | furosta-2α,3β,22α,26-tetrol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 50 | voghieroside E2 | furosta-2α,3β,22β,26-tetrol 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-26-O-α-L-rhamnopyranoside | A. sativum L. var. Voghiera | bulbs | [20] |
| 51 | persicoside D1 | furosta-2α,3β,22ξ,26-tetraol 3-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-β-D-glucopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 52 | persicoside D2 | furosta-2α,3β,22ξ,26-tetraol 3-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-β-D-glucopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 53 | leucofuranoside A | 26-O-β-D-glucopyranosyl-(25R)-5α-furostane-3β,6β-diol-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [22] |
| 54 | (25R)-26-O-β-D-glucopyranosyl-5α-furost-3-β,26-didyroxy-3-O-{O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [16] | |
| 55 | tomatoside A | A. chinense G. Don | bulbs | [16] | |
| 56 | macorstemonoside C | A. chinense G. Don | bulbs | [16] | |
| 57 | (25R)-26-O- β -D-glucopyranosyl-5α -furostane-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | A. chinense G. Don | bulbs | [13] | |
| 58 | dichotomin | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3β, 26-diol-3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 59 | parisaponin | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5-ene-furostan-3β, 26-diol-3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-arabinofuranosyl-(1→4)]-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 60 | persicoside C1 | furosta-1β,3β,22ξ,26-tetraol 5-en 1-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 61 | persicoside C2 | furosta-1β,3β,22ξ,26-tetraol 5-en 1-O-β-D-glucopyranosyl (1→3)-β-D-glucopyranosyl (1→2)-β-D-galactopyranosyl 26-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 62 | ceposide A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 63 | ceposide A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 64 | ceposide C1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 65 | ceposide C2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 66 | tropeoside A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 67 | tropeoside A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 68 | tropeoside B1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 69 | tropeoside B2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 70 | ascalonicoside A1 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 71 | ascalonicoside A2 | A. ampeloprasum subsp. persicum | seeds | [21] | |
| 72 | deltoside | (25R)-furost-5-en-3β,22α,26-triol 26-O-β-D-glucopyranosyl-3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] |
| 73 | karatavioside G | (25R)-26-[(O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl-(1→6)-β-D-glucopyranosyl)oxy]-2α-hydroxy-22α-methoxyfurost-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 74 | allimacroside D | (25R)-26-O-β-D-glucopyranosyl-5-enefurostan-2α,3β,22α,26-tetraol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 75 | tuberoside F | 26-O-β-D-glucopyranosyl-(25S,20R)-20-O-methyl-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 76 | tuberoside G | 26-O-β-D-glucopyranosyl-(25S,20R)-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L- rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 77 | tuberoside H | 26-O-β-D-glucopyranosyl-(25S,20S)-5α-furost-22(23)-en-2α,3β,20,26-tetraol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 78 | tuberoside I | 26-O-β-D-glucopyranosyl-(25S,20S)-5α-furost-22(23)-en-3β,20,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [27] |
| 79 | macrostemonoside L | 26-O-β-D-glucopyranosyl 2β,3β,26-trihydroxy-25(R)-5β-furostan-20(22)-ene 3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [8] |
| 80 | tuberoside A | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 81 | tuberoside B | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 82 | tuberoside C | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-2α,3β,26-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside | A. tuberosum | seeds | [28] |
| 83 | tuberoside R | 26-O-β-D-glucopyranosyl-(25S)-5β-furost-20(22)-ene-2β,3β, 5, 26-tetraol 3-O-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 84 | tuberoside S | 26-O-β-D-glucopyranosyl-(25S)-5β-furost-20(22)-ene-3β,26-diol 3-O-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 85 | tuberoside T | 26-O-β-D-glucopyranosyl-(25S)-5α-furost-20(22)-ene-3β, 26-diol 3-O-α-L-rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 86 | hirtifolioside B | furost-20(22)-ene-2α,3β-diol 3-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyl]-26-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 87 | macrostemonoside E | A. macrostemon Bunge | bulbs | [17] | |
| 88 | macrostemonoside G | 26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-25(27)-ene-3β,12β,26-triol 3-O-β-D-glucopyranosyl(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [30] |
| 89 | macrostemonoside O | 26-O-β-D-glucopyranosyl-22-hydroxy-5-β-furost-25 (27)-ene-3β, 26-diol-3-O-β-D-glucopyranosyl (1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [15] |
| 90 | macrostemonoside N | 22-hydroxy-5β-furost-25-(27)-ene-1β,2β,3β,6α-tetraol-26-O-β-D-glucopyranoside | A. macrostemon Bunge | bulbs | [17] |
| 91 | 26-O-β-D-glucopyranosyl-5α-furost-25 (27)-ene-3β,12β,22,26-tetraol-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [31] | |
| 92 | allimacroside E | 26-O-β-D-glucopyranosyl-20β-methoxyl-25(R)-furostan-5,22(23)-dien-3β,26-diol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 93 | 26-O-β-D-glucopyranosyl-5β-furost-20 (22)-25 (27)-dien-3β,12β,26-triol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [31] | |
| 94 | ascalonicoside C | (25R)-26-O-β-D-glucopyranosyl-22-hydroxy-5α-furost-2-one-3β,5,6β, 26-tetraol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 95 | ascalonicoside D | (25R)-26-O-β-D-glucopyranosyl-22-methoxy-5α-furost-2-one-3β,5,6β, 26-tetraol- 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. ascalonicum L | [23] | |
| 96 | chinenoside I | 26-O-β-D-glucopyranosyl 3β,22,26-tridyroxy-25(R)-5α-furostan-6-one 3-O-β-D-xylopyranosyl(1→4)-[α-L-arabinopyranosyl (1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [32] |
| 97 | 26-O-β-D-glucopyranosyl 3β,22α,26-trihydroxy-25(R)-5α-furostan-6-one | A. chinense G. Don | bulbs | [16] | |
| 98 | 26-O-β-D-glucopyranosyl 3β,22α,26-trihydroxy-25(R)-5α-furostan-6-one 3-O-β-D-glucopyranoside | A. chinense G. Don | bulbs | [16] | |
| 99 | 26-O-β-D-glucopyranosyl 3β,22,26-tridyroxy 25(R)-5α-furostan-6-one 3-O-α-L-arabinopyranosyl(1→6)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [16] | |
| 100 | (25R)-6-ketone-26-O-β-D-glucopyranosyl-5α-furostane-3β,22α,26-triol-3-O-α-L-xylopyranosyl-(1→ 4)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 101 | (25R)-6-ketone-5α -furostane-3β,22α,24β,26-tetraol-3-O-β-D-xylopyranosy-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 102 | chinenoside II | 26-O-β-glucopyranosyl 3β,26-dihydroxy-(25R)-5α-furost-20(22)-en-6-one 3-O-β-xylopyranosyl-(1→4)-[α-arbinopyranosyl(1→6)]-β-glucopyranoside | A. chinense G. Don | bulbs | [33] |
| 103 | chinenoside III | 26-O-β-glucopyranosyl 3β,26-dihydroxy-(25R)-5α-furost-20(22)-en-6-one 3-O-α-arabinopyranosyl(1→6)-β-glucopyranoside | A. chinense G. Don | bulbs | [33] |
| 104 | chinenoside IV | 26-O-β-glucopyranosyl-3β,26-dihydroxy-23-hydroxymethyl-25(R)-5α-furost-20(22)-en-6-one 3-O-β-xylopyranosyl(1→4)-[α-arabinopyranosyl(1→6)]-β-glucopyranoside | A. chinense G. Don | bulbs | [34] |
| 105 | chinenoside V | 26-O-β-glucopyranosyl-3β,26-dihydroxy-23-hydroxymethyl-25(R)-5α-furost-20(22)-en-6-one 3-O-α-arabinopyranosyl(1→6)-β-glucopyranoside | A. chinense G. Don | bulbs | [34] |
| 106 | 26-O-β-D-glucopyranosyl 3β,26-dihydroxy-25(R)-5α-furostan-20(22)-en-6-one | A. chinense G. Don | bulbs | [16] | |
| 107 | macrostemonoside I | 26-O-β-D-glucopyranosyl-22-hydroxy-5β-furost-25(27)-ene12-one-3β,26-diol 3-O-β-D-glucopyranosyl(1→2)-β-D-galactopyranoside | A. macrostemon Bunge | bulbs | [30] |
| 108 | agigenin 3-O-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] | |
| 109 | ampeloside Bs1 | agigenin 3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. ampeloprasum L | bulbs | [3] |
| A. sativum L. var. Voghiera | bulbs | [20] | |||
| 110 | desgalactotigonin | A. sativum L | roots | [4] | |
| 111 | F-gitonin | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. sativum L | roots | [4] |
| A. ostrowskianum | bulbs | [5] | |||
| A. jesdianum | bulbs | [35] | |||
| A. porrum L | bulbs | [36] | |||
| flowers | [37] | ||||
| A. cyrillii | bulbs | [38] | |||
| 112 | sativoside-R2 | tigogenin 3-O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranosyde | A. sativum L | roots | [4] |
| 113 | (24S,25R)-5α-spirostan-2α,3β,5α,6β,24-pentaol 24-O-β-D-glucopyranoside | A. giganteum | bulbs | [39] | |
| 114 | aginoside | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. giganteum | bulbs | [39] |
| A. albopilosum | bulbs | [5] | |||
| A. ostrowskianum | bulbs | [5] | |||
| A. schubertii | bulbs | [7] | |||
| A. macleanii | bulbs | [40] | |||
| A. ampeloprasum L | bulbs | [41] | |||
| A. jesdianum | bulbs | [35] | |||
| A. leucanthum | flowers | [42] | |||
| A. nigrum L | bulbs | [43] | |||
| root–bulb basal stem | [44] | ||||
| A. porrum L | flowers | [37] | |||
| 115 | (25R)-5α-spirostan-2α,3β,5α,6α-tetraol 2-O-β-D-glucopyranoside | A. aflatunense | bulbs | [39] | |
| 116 | alliogenin | (25R)-5α-spirostan-2α,3β,5α,6β-tetraol | A. giganteum | bulbs | [45] |
| A. albopilosum | bulbs | [5] | |||
| A. karataviense | bulbs | [9] | |||
| A. minutiflorum Regel | bulbs | [14] | |||
| 117 | (25R)-3-O-acetyl-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. albopilosum | bulbs | [5] | |||
| A. karataviense | bulbs | [9] | |||
| 118 | (25R)-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. albopilosum | bulbs | [5] | |||
| A. macleanii | bulbs | [40] | |||
| A. karataviense | bulbs | [9] | |||
| 119 | (25R)-3-O-benzoyl-5α-spirostan-2α,3β,5α,6β-tetraol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [45] | |
| A. macleanii | bulbs | [40] | |||
| A. karataviense | bulbs | [9] | |||
| 120 | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-(O-β-D-glucopyranosyl-(1→2)-O-[3-O-acetyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 121 | (25S)-5α-spirostane-2α,3β,6β-triol 3-O-(O-β-D-glucopyranosyl-(1→2)-O-[3-O-acetyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 122 | (25R)-2-O-[(S)-3-hydroxy-3-methylglutaroyl]-5α-spirostane-2α,3β,6β-triol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. albopilosum | bulbs | [5] | |
| 123 | turoside A | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] |
| A. nigrum L | bulbs | [43] | |||
| root–bulb basal stem | [44] | ||||
| 124 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. macleanii | bulbs | [40] | |||
| 125 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 126 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[3-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 127 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[3-O-benzoyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| 128 | (25R)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-(3S)-3-hydroxy-3-methylglutaroyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. giganteum | bulbs | [6] | |||
| A. nigrum L | bulbs | [43] | |||
| 129 | (25S)-5α-spirostan-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-O-[4-O-(3S)-3-hydroxy-3-methylglutaroyl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. schubertii | bulbs | [7] | |
| A. nigrum L | bulbs | [43] | |||
| 130 | 3-O-acetyl-(24S,25S)-5α-spirostane-2α,3β,5α,6β,24-pentol 2-O-β-D-glucopyranoside | A. giganteum | bulbs | [6] | |
| 131 | methyl ester of (25R)-5α-spirostane-2α,3β,6β-triol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[4-O-(S)-3-hydroxy-3-methylglutaryl-β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. macleanii | bulbs | [40] | |
| 132 | tigogenin 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-β-D-xylopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. macleanii | bulbs | [40] | |
| 133 | macrostemonoside A | (25R)-5α-spirostan-3β-ol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyraoside} | A. chinense G. Don | bulbs | [46] |
| A. macrostemon Bunge | bulbs | [47] | |||
| 134 | (25S)-5α-spirostan-3β-ol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyraoside} | A. chinense G. Don | bulbs | [46] | |
| 135 | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| A. sativum L. var. Voghiera | bulbs | [20] | |||
| 136 | (25S)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 137 | (25R)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 138 | (25S)-5α-spirostane-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 139 | agigenin | spirostan-2α, 3β, 6β–triol | A. porrum L | bulbs | [48] |
| flowers | [37] | ||||
| 140 | neoagigenin | A. porrum L | bulbs | [48] | |
| A. minutiflorum Regel | bulbs | [14] | |||
| 141 | porrigenin A | (25R)-5α-spirostan-2β,3β,6β-triol | A. porrum L | bulbs | [48] |
| 142 | neoporrigenin A | (25S)-5α-spirostan-2β,3β,6β-triol | A. porrum L | bulbs | [48] |
| 143 | yayoisaponin A | agigenin 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 144 | yayoisaponin C | agigenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 145 | timosaponin A III | sarsasapogenin 3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 146 | macrostemonoside D | tigogenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-(6-acetyl-β-D-glucopyranosyl)-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 147 | neomacrostemonoside D | neotigogenin 3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-(6-acetyl-β-D-glucopyranosyl)-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [49] |
| 148 | alliogenin 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. karataviense | bulbs | [9] | |
| 149 | (25R)-3-O-(2-hydroxybutyryl)-5α-spirostane-2α,3β,5,6β-tetrol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 150 | (24S,25S)-3-O-benzoyl-5α-spirostane-2α,3β,5,6β,24-pentol 2-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 151 | (24S,25S)-5α-spirostane-2α,3β,5,6β,24-pentol 2,24-di-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 152 | (24S,25S)-3-O-benzoyl-5α-spirostane-2α,3β,5,6β,24-pentol 2,24-di-O-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 153 | (24S,25S)-5α-spirostane-2α,3β,5,6β,24-pentol 2-O-β-D-glucopyranosyl 24-O-{O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside | A. karataviense | bulbs | [9] | |
| 154 | (25R)-5α-spirostan-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| 155 | (25R)-5α-spirostan-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| A. rotundum | inflorescences and flower stalks | [19] | |||
| 156 | (25R)-5α-spirostan-3β,6β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| A. leucanthum | flowers | [42] | |||
| 157 | (25R)-5α-spirostan-3β,6β-diol 3-O-{O-β-D-glucopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [50] | |
| 158 | tuberoside D | (25S)-5α-spirostane-2α,3β‚-diol 3-O-α-L-rhamnopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-O-β-D-glucopyranoside | A. tuberosum | seeds | [51] |
| 159 | tuberoside E | (25S)-5α-spirostan-2α,3β-diol 3-O-β-D-glucopyranosyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]O-β-D-glucopyranoside | A. tuberosum | seeds | [51] |
| 160 | (25S)-spirostane-3β,5β,6α-triol 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum | seeds | [52] | |
| 161 | (25S)-5β-spirostane-3β,6α-diol (25epi-ruizgenin) 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranoside | A. tuberosum | seeds | [52] | |
| 162 | tuberoside J | (25R)-5α-spirostan-2α,3β,27-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 163 | tuberoside K | (25R)-5α-spirostan-2α,3β,27-triol 3-O-α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 164 | tuberoside L | 27-O-β-D-glucopyranosyl-(25R)-5α-spirostan-2α,3β,27-triol 3-O-α-D-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum | seeds | [53] |
| 165 | tuberoside | (2α,3β,5α,25S)-2,3,27-trihydroxyspirostane 3-O-α-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranoyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [54] |
| 166 | tuberoside N | (25S)-5β-spirostan-2β,3β-diol 3-O-β-D-glucopyranosyl-(1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 167 | tuberoside O | (25S)-5β-spirostan-2β,3β,5-triol 3-O-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| roots | [55] | ||||
| 168 | tuberoside P | (25S)-5β-spirostan-2β,3β, 5-triol 3-O-α-L-rhamnopyranosyl (1→4)-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 169 | tuberoside Q | (24S,25S)-5β-spirostan-2β,3β,5,24-tetraol 3-O-α-L-rhamnopyranosyl (1→4)-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 170 | agapanthagenin | A. elburzense | bulbs | [10] | |
| 171 | hirtifolioside D | spirostan-2α,3β,6β-triol 3-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. hirtifolium Boiss | flowers | [12] |
| 172 | agapanthagenin 3-O-β-D-glucopyranoside | A. hirtifolium Boiss | flowers | [12] | |
| 173 | atroviolacegenin | (25R)-5α-spirostan-2α,3β,6β,27-tetrol | A. atroviolaceum | flowers | [56] |
| 174 | atroviolaceoside | (25R)-5α-spirostan-2α,3β,6β,27-tetrol 3-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. atroviolaceum | flowers | [56] |
| 175 | minutoside B | (25S)-spirostan-2α,3β,6β-triol 3-O-β-D-xylopyranosyl-(1→3)-O-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. minutiflorum Regel | bulbs | [14] |
| 176 | eruboside B | β-chlorogenin 3-O-β-glucopyranosyl(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl(1→4)-β-galactopyranoside | A. leucanthum | flowers | [42] |
| A. sativum L | bulbs | [2] | |||
| 177 | leucospiroside A | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-β-glucopyranosyl-(1→3)-β-glucopyranosyl-(1→2)-[β-glucopyranosyl-(1→3)]-β-glucopyranosyl-(1→4)-β-galactopyranoside | A. leucanthum | flowers | [42] |
| A. ampeloprasum var. porrum | bulbs | [57] | |||
| 178 | (25R)-5α-spirostane-2α,3β,6β-triol 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [42] | |
| 179 | (25R)-5α-spirostane-3β,6β-diol 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. leucanthum | flowers | [42] | |
| 180 | tuberoside A | (24S, 25S)-5β-spirostan-2β,3β,24-triol 3-O-a-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [58] |
| 181 | (3β,5α,6β,25R)-6-[(β-D-glucopyranosyl)oxy]spirostan-3-yl O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-β-D-galactopyranoside | A. ampeloprasum var. porrum | bulbs | [59] | |
| 182 | nigroside A1 | 25(R)-5α-spirostan-2α,3β,6β-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. nigrum L | bulbs | [43] |
| 183 | nigroside A2 | 25(S)-5α-spirostan-2α,3β,6β-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. nigrum L | bulbs | [43] |
| 184 | nigroside B1 | 25(R)-5α-spirostan-2α,3β,6β-trio 1-2-O-[β-D-glucopyranosyl]-3-O-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 185 | nigroside B2 | 25(S)-5α-spirostan-2α,3β,6β-trio 1-2-O-[β-D-glucopyranosyl]-3-O-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 186 | β-D-glucopyranosyl-(1→2)-[4-O-(3-hydroxy-3-methylglutaryl)-β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl-(1→3)-(25R)-5α-spirostan-2α,3β-diol | A. cyrillii | bulbs | [38] | |
| 187 | persicoside A | (25S)-spirostan-2α,3β,6β-triol 3-O-[β-D-glucopyranosyl-(1→3)] [β-D-xylopyranosyl-(1→2)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 188 | persicoside B | (25S)-spirostan-2α,3β,6β-triol 3-O-[β-D-xylopyranosyl-(1→3)] [α-L-rhamnopyranosyl(1→2)]-β-D-glucopyranosyl-(1→4)-O-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 189 | (25R)-5α-spirostan-3β,11aα-diol 3-O-β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→4)]-β-D-galactopyranoside | A. schoenoprasum | whole plants | [24] | |
| 190 | tuberoside B | (24S,25S)-5β-spirostan-2α,3β,5,24-tetraol 3-O-α-L-rhamnopyranoyl-(1→2)-O-[α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyranoside | A. tuberosum Rottl. ex Spreng | seeds | [60] |
| 191 | tuberosine A | (25S)-5β-spirostan-2β,3β-diol 3-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 192 | tuberosine B | (25S)-5β-spirostan-2β,3β,19-triol 3-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 193 | tuberosine C | (25S)-5β-spirostan-2β,3β-diol 3-O-α-L-rhamnopyranoyl-(1→4)-O-β-D-glucopyranoside | A. tuberosum | roots | [55] |
| 194 | 25(S)-schidigera-saponin D5 | A. tuberosum | roots | [55] | |
| 195 | shatavarin IV | A. tuberosum | roots | [55] | |
| 196 | karatavioside I | (24S,25S)-24-[(O-β-D-glucopyranosyl(1→2)-β-D-glucopyranosyl)oxy]-2α,5α,6β-trihydroxyspirostan-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 197 | neogitogenin | A. chinense G. Don | bulbs | [61] | |
| 198 | (25R)-5α-spirostan-3β-yl-3-O-acetyl-O-β-D-glucopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. chinense G. Don | bulbs | [61] | |
| 199 | (25S)-5α-spirostane-3β-ol-3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 200 | allimacroside B | (24S,25S)-24-[(β-D-glucopyranosyl)oxy]-5α-spirostan-3β-yl-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 201 | yayoisaponin A/alliporin | (2α, 3β, 6β, 25R)-2,6-dihydroxyspirostan-3-yl β-D-glucopyranosyl-(1→3)-β-D-glucopranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl]-(1→4)-β-D-galactopyranoside | A. porrum L | flowers | [37] |
| 202 | alliospiroside A | (25S)-3β-hydroxyspirost-5-en-1β-yl-2-O-(6-deoxy-α-L-mannopyranosyl)-α-L-arabinopyranoside | A. cepa L | collective fruit | [62] |
| A. cepa L. Aggregatum group | roots | [63] | |||
| 203 | alliospiroside B | A. cepa L | collective fruit | [62] | |
| 204 | alliospiroside D | A. cepa L | collective fruit | [62] | |
| 205 | (25R)-spirost-5-en-3β-ol (diosgenin) 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-[O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)]-β-D-glucopyanoside} | A. senescens | bulbs | [40] | |
| 206 | diosgenin 3-O-{O-α-L-rhamnopyranosyl-(1→2)-O-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranoside} | A. senescens | bulbs | [40] | |
| 207 | dioscin | A. ampeloprasum L | bulbs | [41] | |
| 208 | (25R)-spirost-5-ene-2α,3β-diol 3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. karataviense | bulbs | [9] | |
| 209 | β-chacotriosyl lilagenin | A. tuberosum | seeds | [52] | |
| 210 | (20S,25S)-spirost-5-en-3β,12β,21-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 211 | (20S,25S)-spirost-5-en-3β,11α,21-triol 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 212 | diosgenin 3-O-α-L-rhamnopyranosyl-(1→2)-O-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 213 | deltonin | diosgenin 3-O-β-D-glucopyranosyl-(1→ 4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] |
| 214 | karatavioside H | (24S,25S)-24-[(O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranosyl) oxy]-2α-hydroxyspirost-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 215 | allimacroside C | (24S,25S)-24-[(β-D-glucopyranosyl)oxy]-spirost-5-ene-3β,24-diol-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-D-glucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
| 216 | 5α-spirostane 25(27)-ene-2α,3β-diol-3-O-{O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 217 | porrigenin B | (25R)-2-oxo-5α-spirostan-3β,6β-diol | A. porrum L | bulbs | [48] |
| 218 | neoporrigenin B | (25S)-2-oxo-5α-spirostan-3β,6β-diol | A. porrum L | bulbs | [48] |
| 219 | yayoisaponin B | porrigenin B 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. ampeloprasum L | bulbs | [41] |
| 220 | (3β,5α,6β,25R)-3-{(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-O-[O-β-D-glucopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranosyl)oxy}-6-hydroxyspirostan-2-one | A. ampeloprasum var. porrum | bulbs | [64] | |
| 221 | 3-keto umbilicagenin A | (25R)-3-keto-spirostan-2α,5α,6β-triol | A. umbilicatum Boiss | flowers | [65] |
| 222 | 3-keto umbilicagenin B | (25R)-3-keto-spirostan-2α,5α-diol | A. umbilicatum Boiss | flowers | [65] |
| 223 | anzurogenin A | 2α,3β,5β-trihydroxy-(25R)-spirostan-6-one | A. suvorovii and A. stipitatum | fruit | [66] |
| 224 | anzuroside | (24S, 25S)-2α,3β,5,24-tetrahydroxy-5β-spirostan-6-one 24-O-β-D-glucopyranoside | A. suvorovii and A. stipitatum | fruits | [67] |
| 225 | anzurogenin C | (24S, 25S)-2α,3β,5,24-tetrahydroxy-5β-spirostan-6-one | A. suvorovii and A. stipitatum | fruits | [67] |
| A. chinense G. Don | bulbs | [49] | |||
| 226 | (25R)-3β-hydroxy-5α-spirostan-6-one (laxogenin) 3-O-{O-α-L-arabinopyranosyl-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 227 | laxogenin 3-O-{O-(2-O-acetyl-α-L-arabinopyranosyl)-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] | |
| 228 | xiebai-saponin I | laxogenin 3-O-{O-β-D-xylopyranosyl-(1→4)-O-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [46] |
| 229 | chinenosideVI | (25R)-24-O-β-D-glucopyranosyl-3β,24β-dihydroxy-5α-spirost 3-O-α-arabinopyranosyl-(1→6)-β-D-glucopyranoside | A. chinense G. Don | bulbs | [49] |
| 230 | laxogenin 3-O-β-D-glucopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [49] | |
| 231 | laxogenin | A. chinense G. Don | bulbs | [68] | |
| 232 | laxogenin 3-O-α-L-rhamnopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→4)]-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 233 | laxogenin 3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside | A. schoenoprasum | whole plants | [24] | |
| 234 | laxogenin 3-O-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 235 | laxogenin 3-O-{β-D-xylopyranosyl-(1→4)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 236 | (25R)-5α-spirostan 3-O-{O-(4-O-acetyl-α-L-arabinopyranosyl)-(1→6)-β-D-glucopyranoside} | A. chinense G. Don | bulbs | [61] | |
| 237 | (25R)-3β-hydroxy-5β-spirostan-6-one 3-O-β-D-xylopyranosyl(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 238 | (25R)-3β-hydroxy-5α-spirostan-6-one 3-O-{[O-β-D-glucopyranosyl-(1→3)-O-β-D-xylopyranosyl]-(1→4)-O-[α-L-arabinopyranosyl-(1→6)]}-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 239 | (25S)-3β,24β-dihydroxy-5α-spirostan-6-one 3-O-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [61] | |
| 240 | (25S)-24-O-β-D-glucopyranosyl-3β,24β-dihydroxy-5α-spirostan-6-one | A. chinense G. Don | bulbs | [61] | |
| 241 | 12-keto-porrigenin | (25R)-5α-spirostan-3β, 6β-diol-12-one | A. porrum L | [69] | |
| 242 | (25S)-5α-spirostan-3β, 6β-diol-12-one | A. porrum L | [69] | ||
| 243 | (25R)-3β,6β‚dihydroxy-5α-spirostan-12-one-3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [36] | |
| 244 | (25R)-5α-spirostan-3β,6β-diol-12-one 3-O-β-D-glucopyranosyl-(1→2)-[β-D-fucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. porrum L | bulbs | [70] | |
| 245 | porrigenin C | A. porrum L | [71] | ||
| 246 | neoporrigenin C | A. porrum L | [71] | ||
| 247 | (25R)-3β,6β-dihydroxy-5α-spirostan-2,12-dione-3-O-{O-β-D-glucopyranosyl-(1→2)-O-[β-D-xylopyranosyl-(1→3)]-O-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [36] | |
| 248 | (25R)-5α-spirostane-3β,6β-diol-2,12-dione 3-O-{β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-(1→2)-[β -D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside} | A. porrum L | bulbs | [72] | |
| 249 | (25R)-spirost-4-ene-3-one-2-ol | A. fistulosum L | seeds | [73] | |
| 250 | (25R)-spirost-1,4-diene-3-one-2,6-diol | A. fistulosum L | seeds | [73] | |
| 251 | (25R)-spirost-1,4-diene-3-one-2-ol | A. fistulosum L | seeds | [73] | |
| 252 | (25R)-19-norspirosta-1,3,5 (10)-triene-4-methyl-2-ol | A. fistulosum L | seeds | [73] | |
| 253 | anzurogenin B | 2α,5α-epoxy-(25R)-spirostan-3β,6β-diol | A. suvorovii and A. stipitatum | fruit | [74] |
| 254 | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 255 | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 16-O-{O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranoside} | A. ostrowskianum | bulbs | [5] | |
| 256 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1,3-di-O,O’-α-L-rhamnopyranoside 16-O-β-D-glucopyranoside | A. macleanii | bulbs | [40] | |
| 257 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1,16-di-O-β-D-glucopyranoside | A. jesdianum | bulbs | [35] | |
| 258 | (22S)-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-α-L-rhamnopyranosyl 16-O-β-D-glucopyranoside | A. jesdianum | bulbs | [35] | |
| 259 | 22S-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-α-L-rhamnopyranosyl 16-O-β-D-galactopyranoside | A. porrum L | bulbs | [36] | |
| 260 | 22S-cholest-5-ene-1β,3β,16β,22-tetrol 1-O-[O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranoside] 16-O-β-D-galactopyranoside | A. porrum L | bulbs | [36] | |
| 261 | tuberoside U | 16-O-β-D-glucopyranosyl-(22S,25S)-cholest-5-ene-3β,16β, 22, 26-tetraol 3-O-α-L-rhamnopyranosyl (1→2)-[α-L-rhamnopyranosyl (1→4)]-β-D-glucopyranoside | A. tuberosum L | seeds | [29] |
| 262 | nigroside C | (22S)-cholest-5-ene-1β,3β,16β, 22-tetraol 1-O-[α-L-rhamnopyranosyl] 16-O-α-L-rhamnopyranosyl-(1→3)-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 263 | nigroside D | (22S)-cholest-5-ene-1β,3β,16β,22-tetraol 16-O-α-L-rhamnopyranosyl-(1→3)-β-D-galactopyranoside | A. nigrum L | bulbs | [43] |
| 264 | persicoside E | (22S)-cholesta-1β,3β,16β,22β-tetraol 5-en 1-O-α-L-rhamnopyranosyl 16-O-α-L-rhamnopyranosyl (1→2)-β-D-galactopyranoside | A. ampeloprasum subsp. persicum | seeds | [21] |
| 265 | karatavioside J | (22S)-16β-[(β-D-glucopyranosyl)oxy]-22-hydroxycholest-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→ 4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 266 | karatavioside K | (22S)-16β-[(O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl)oxy]-22-hydroxycholest-5-en-3β-yl O-β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside | A. karataviense | bulbs | [25] |
| 267 | 1β,3β,16β-trihydroxy-5α-cholestan-22-one 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 268 | 1β,3β,16β-trihydroxycholest-5-en-22-one 1-O-α-L-rhamnopyranoside 16-O-{O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside} | A. albopilosum | bulbs | [5] | |
| 269 | 2,3-seco-porrigenin | (25R)-5α-2,3-secospirostan-2,3-dioic acid-6β-hydroxy-3,6-γ-lactone | A. porrum L | [69] | |
| 270 | (25S)-5α-2,3-secospirostan-2,3-dioic acid-6β-hydroxy-3,6-γ-lactone | A. porrum L | [69] | ||
| 271 | 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl 3β,5β,6α,16β-tetrahydroxypregnane 16-(5-O-β-D-glucopyranoyl-4(S)-methyl-5-hydroxypentanoic acid) ester | A. tuberosum Rottler | seeds | [11] | |
| 272 | 5α-cholano-22,16-lactone-3-O-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galacopyranoside | A. chinense G. Don | bulbs | [13] | |
| 273 | 6-ketone-5α-cholano-22,16-lactone-3-O-β-D-6-xylopyranosyl-(1→4)-[α-L-arabinopyranosyl-(1→6)]-β-D-glucopyranoside | A. chinense G. Don | bulbs | [13] | |
| 274 | allimacroside A | pregna-5,16-dien-3β-ol-20-one-3-O-β-D-glucopyranosyl(1→2)-[β-D-glucopyranosyl(1→3)]-β-Dglucopyranosyl(1→4)-β-D-galactopyranoside | A. macrostemon Bunge | whole plants | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zheng, Q.; Dong, A.; Wang, J.; Si, J. Chemical Constituents, Biological Activities, and Proposed Biosynthetic Pathways of Steroidal Saponins from Healthy Nutritious Vegetable—Allium. Nutrients 2023, 15, 2233. https://doi.org/10.3390/nu15092233
Wang H, Zheng Q, Dong A, Wang J, Si J. Chemical Constituents, Biological Activities, and Proposed Biosynthetic Pathways of Steroidal Saponins from Healthy Nutritious Vegetable—Allium. Nutrients. 2023; 15(9):2233. https://doi.org/10.3390/nu15092233
Chicago/Turabian StyleWang, Huaxiang, Qi Zheng, Aijun Dong, Junchi Wang, and Jianyong Si. 2023. "Chemical Constituents, Biological Activities, and Proposed Biosynthetic Pathways of Steroidal Saponins from Healthy Nutritious Vegetable—Allium" Nutrients 15, no. 9: 2233. https://doi.org/10.3390/nu15092233