Longitudinal Associations of Dietary Sugars and Glycaemic Index with Indices of Glucose Metabolism and Body Fatness during 3-Year Weight Loss Maintenance: A PREVIEW Sub-Study
Abstract
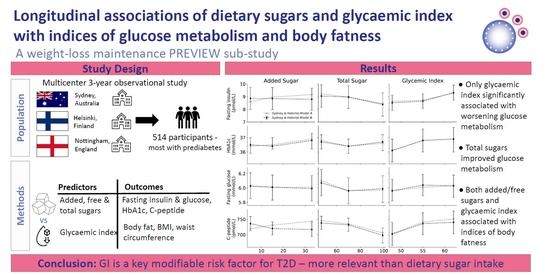
:1. Introduction
2. Participants and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Indices of Glucose Metabolism
3.2. Indices of Body Fatness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.-P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Imamura, F.; Lentjes, M.A.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia 2015, 58, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Tsilas, C.S.; de Souza, R.J.; Mejia, S.B.; Mirrahimi, A.; Cozma, A.I.; Jayalath, V.H.; Ha, V.; Tawfik, R.; Di Buono, M.; Jenkins, A.L. Relation of total sugars, fructose and sucrose with incident type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. CMAJ 2017, 189, E711–E720. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.F.; Buyken, A.E.; Jenkins, D.J.; Augustin, L.S.; Sievenpiper, J.L.; Barclay, A.W.; Liu, S.; Wolever, T.M. Dietary glycemic index and load and the risk of type 2 diabetes: A systematic review and updated meta-analyses of prospective cohort studies. Nutrients 2019, 11, 1280. [Google Scholar] [CrossRef]
- Hu, F.B.; Malik, V.S. Sugar-sweetened beverages and risk of obesity and type 2 diabetes: Epidemiologic evidence. Physiol. Behav. 2010, 100, 47–54. [Google Scholar] [CrossRef]
- Siervo, M.; Montagnese, C.; Mathers, J.C.; Soroka, K.R.; Stephan, B.C.; Wells, J.C. Sugar consumption and global prevalence of obesity and hypertension: An ecological analysis. Public Health Nutr. 2014, 17, 587–596. [Google Scholar] [CrossRef]
- DiMeglio, D.P.; Mattes, R.D. Liquid versus solid carbohydrate: Effects on food intake and body weight. Int. J. Obes. 2000, 24, 794–800. [Google Scholar] [CrossRef]
- Sievenpiper, J.L.; de Souza, R.J.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Beyene, J.; Chiavaroli, L.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A. Effect of fructose on body weight in controlled feeding trials: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 156, 291–304. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2013, 346, e7492. [Google Scholar] [CrossRef] [PubMed]
- Saris, W.H.; Astrup, A.; Prentice, A.M.; Zunft, H.J.; Formiguera, X.; Verboeket-van de Venne, W.P.; Raben, A.; Poppitt, S.D.; Seppelt, B.; Johnston, S.; et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The CARMEN study. The Carbohydrate Ratio Management in European National diets. Int. J. Obes. 2000, 24, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Liu, S.; Manson, J.E.; Hu, F.B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 2008, 87, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, D.C.; Threapleton, D.E.; Evans, C.E.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Burley, V.J. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: Systematic review and dose–response meta-analysis of prospective studies. Diabetes Care 2013, 36, 4166–4171. [Google Scholar] [CrossRef]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef]
- Farvid, M.S.; Homayouni, F.; Shokoohi, M.; Fallah, A.; Farvid, M.S. Glycemic index, glycemic load and their association with glycemic control among patients with type 2 diabetes. Eur. J. Clin. Nutr. 2014, 68, 459–463. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 699–706. [Google Scholar] [CrossRef]
- Ojo, O.; Ojo, O.O.; Adebowale, F.; Wang, X.-H. The effect of dietary glycaemic index on glycaemia in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2018, 10, 373. [Google Scholar] [CrossRef]
- Kristo, A.S.; Matthan, N.R.; Lichtenstein, A.H. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: Review of randomized controlled-feeding trials. Nutrients 2013, 5, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M.; Larsen, T.M.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Boyadjieva, N.; Poppitt, S.; Schlicht, W.; Stratton, G.; Sundvall, J.; et al. PREVIEW: Prevention of diabetes through lifestyle intervention and population studies in Europe and around the world. design, methods, and baseline participant description of an adult cohort enrolled into a three-year randomised clinical trial. Nutrients 2017, 9, 632. [Google Scholar] [CrossRef]
- Zhu, R.; Larsen, T.M.; Fogelholm, M.; Poppitt, S.D.; Vestentoft, P.S.; Silvestre, M.A.-O.; Jalo, E.; Navas-Carretero, S.; Huttunen-Lenz, M.; Taylor, M.A.; et al. Dose-Dependent Associations of Dietary Glycemic Index, Glycemic Load, and Fiber With 3-Year Weight Loss Maintenance and Glycemic Status in a High-Risk Population: A Secondary Analysis of the Diabetes Prevention Study PREVIEW. Diabetes Care 2021, 44, 1672–1681. [Google Scholar] [CrossRef]
- Walton, J.A.-O.; Bell, H.; Re, R.; Nugent, A.A.-O. Current perspectives on global sugar consumption: Definitions, recommendations, population intakes, challenges and future direction. Nutr. Res. Rev. 2021, 1–22. [Google Scholar] [CrossRef]
- Raben, A.A.-O.; Vestentoft, P.A.-O.; Brand-Miller, J.A.-O.; Jalo, E.A.-O.; Drummen, M.A.-O.X.; Simpson, L.A.-O.; Martinez, J.A.-O.; Handjieva-Darlenska, T.A.-O.X.; Stratton, G.A.-O.; Huttunen-Lenz, M.A.-O.; et al. The PREVIEW intervention study: Results from a 3-year randomized 2 × 2 factorial multinational trial investigating the role of protein, glycaemic index and physical activity for prevention of type 2 diabetes. Diabetes Obes. Metab. 2021, 23, 324–337. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Care in Diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef]
- Wolever, T.M.; Yang, M.; Zeng, X.Y.; Atkinson, F.; Brand-Miller, J.C. Food glycemic index, as given in glycemic index tables, is a significant determinant of glycemic responses elicited by composite breakfast meals. Am. J. Clin. Nutr. 2006, 83, 1306–1312. [Google Scholar] [CrossRef]
- Louie, J.C.Y.; Flood, V.M.; Atkinson, F.S.; Barclay, A.W.; Brand-Miller, J.C. Methodology for assigning appropriate glycaemic index values to an Australian food composition database. J. Food Compos. Anal. 2015, 38, 1–6. [Google Scholar] [CrossRef]
- van Woudenbergh, G.J.; Kuijsten, A.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C.; Feskens, E.J. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J. Nutr. Metab. 2011, 2011. [Google Scholar] [CrossRef]
- Wanselius, J.; Axelsson, C.; Moraeus, L.; Berg, C.; Mattisson, I.; Larsson, C. Procedure to Estimate Added and Free Sugars in Food Items from the Swedish Food Composition Database Used in the National Dietary Survey Riksmaten Adolescents. Nutrients 2019, 11, 1342. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Carbohydrate intake and incidence of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Br. J. Nutr. 2008, 99, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Villegas, R.; Liu, S.; Gao, Y.-T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Janket, S.-J.; Manson, J.E.; Sesso, H.; Buring, J.E.; Liu, S. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003, 26, 1008–1015. [Google Scholar] [CrossRef]
- Ahmadi-Abhari, S.; Luben, R.N.; Powell, N.; Bhaniani, A.; Chowdhury, R.; Wareham, N.J.; Forouhi, N.G.; Khaw, K.-T. Dietary intake of carbohydrates and risk of type 2 diabetes: The European Prospective Investigation into Cancer-Norfolk study. Br. J. Nutr. 2014, 111, 342–352. [Google Scholar] [CrossRef]
- Della Corte, K.A.; Penczynski, K.; Kuhnle, G.; Perrar, I.; Herder, C.; Roden, M.; Wudy, S.A.; Remer, T.; Alexy, U.; Buyken, A.E. The prospective association of dietary sugar intake in adolescence with risk markers of type 2 diabetes in young adulthood. Front. Nutr. 2021, 7, 615684. [Google Scholar] [CrossRef]
- Montonen, J.; Järvinen, R.; Knekt, P.; Heliovaara, M.; Reunanen, A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J. Nutr. 2007, 137, 1447–1454. [Google Scholar] [CrossRef]
- Wu, T.; Giovannucci, E.; Pischon, T.; Hankinson, S.E.; Ma, J.; Rifai, N.; Rimm, E.B. Fructose, glycemic load, and quantity and quality of carbohydrate in relation to plasma C-peptide concentrations in US women. Am. J. Clin. Nutr. 2004, 80, 1043–1049. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363–375. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Dashti, H.S.; Ma, J.; Haslam, D.E.; Kiefte-de Jong, J.C.; Smith, C.E.; Tanaka, T.; Graff, M.; Lemaitre, R.N.; Rybin, D. Sugar-sweetened beverage intake associations with fasting glucose and insulin concentrations are not modified by selected genetic variants in a ChREBP-FGF21 pathway: A meta-analysis. Diabetologia 2018, 61, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose–response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef]
- Colditz, G.A.; Manson, J.; Stampfer, M.J.; Rosner, B.; Willett, W.C.; Speizer, F.E. Diet and risk of clinical diabetes in women. Am. J. Clin. Nutr. 1992, 55, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Feskens, E.J.; Virtanen, S.M.; Räsänen, L.; Tuomilehto, J.; Stengård, J.; Pekkanen, J.; Nissinen, A.; Kromhout, D. Dietary factors determining diabetes and impaired glucose tolerance: A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995, 18, 1104–1112. [Google Scholar] [CrossRef]
- Barclay, A.W.; Flood, V.M.; Rochtchina, E.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, dietary fiber, and risk of type 2 diabetes in a cohort of older Australians. Diabetes Care 2007, 30, 2811–2813. [Google Scholar] [CrossRef]
- Sluijs, I.; Van Der Schouw, Y.T.; Van Der A, D.L.; Spijkerman, A.M.; Hu, F.B.; Grobbee, D.E.; Beulens, J.W. Carbohydrate quantity and quality and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) study. Am. J. Clin. Nutr. 2010, 92, 905–911. [Google Scholar] [CrossRef]
- Heikkilä, H.; Schwab, U.; Krachler, B.; Männikkö, R.; Rauramaa, R. Dietary associations with prediabetic states—The DR’s EXTRA study (ISRCTN45977199). Eur. J. Clin. Nutr. 2012, 66, 819–824. [Google Scholar] [CrossRef]
- dden Biggelaar, L.J.; Eussen, S.J.; Sep, S.J.; Mari, A.; Ferrannini, E.; van Dongen, M.C.; Denissen, K.F.; Wijckmans, N.E.; Schram, M.T.; van der Kallen, C.J. Associations of dietary glucose, fructose, and sucrose with β-cell function, insulin sensitivity, and type 2 diabetes in the Maastricht study. Nutrients 2017, 9, 380. [Google Scholar] [CrossRef]
- Tasevska, N.; Pettinger, M.; Kipnis, V.; Midthune, D.; Tinker, L.F.; Potischman, N.; Neuhouser, M.L.; Beasley, J.M.; Van Horn, L.; Howard, B.V. Associations of biomarker-calibrated intake of total sugars with the risk of type 2 diabetes and cardiovascular disease in the Women’s Health Initiative Observational Study. Am. J. Epidemiol. 2018, 187, 2126–2135. [Google Scholar] [CrossRef]
- Weber, K.S.; Simon, M.-C.; Strassburger, K.; Markgraf, D.F.; Buyken, A.E.; Szendroedi, J.; Müssig, K.; Roden, M.; Group, G. Habitual fructose intake relates to insulin sensitivity and fatty liver index in recent-onset type 2 diabetes patients and individuals without diabetes. Nutrients 2018, 10, 774. [Google Scholar] [CrossRef]
- Kim, H.S.; Paik, H.Y.; Lee, K.U.; Lee, H.K.; Min, H.K. Effects of several simple sugars on serum glucose and serum fructose levels in normal and diabetic subjects. Diabetes Res. Clin. Pract. 1988, 4, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; van der A, D.L.; van Bakel, M.M.; van der Kallen, C.J.; Blaak, E.E.; van Greevenbroek, M.M.; Jansen, E.H.; Nijpels, G.; Stehouwer, C.D.; Dekker, J.M. index and glycemic load in relation to food and nutrient intake and metabolic risk factors in a Dutch population. Am. J. Clin. Nutr. 2008, 87, 655–661. [Google Scholar] [CrossRef]
- Bhupathiraju, S.N.; Tobias, D.K.; Malik, V.S.; Pan, A.; Hruby, A.; Manson, J.E.; Willett, W.C.; Hu, F.B. Glycemic index, glycemic load, and risk of type 2 diabetes: Results from 3 large US cohorts and an updated meta-analysis. Am. J. Clin. Nutr. 2014, 100, 218–232. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Zhang, L.; Zhang, Y.-H.; Qin, L.-Q. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2011, 106, 1649–1654. [Google Scholar] [CrossRef]
- Hardy, D.S.; Garvin, J.T.; Xu, H. Carbohydrate quality, glycemic index, glycemic load and cardiometabolic risks in the US, Europe and Asia: A dose–response meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 853–871. [Google Scholar] [CrossRef]
- WHO. Sugars Intake for Adults and Children Geneva; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Bray, G.A. Soft drink consumption and obesity: It is all about fructose. Curr. Opin. Lipidol. 2010, 21, 51–57. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; O’Keefe, J.H. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018, 61, 3–9. [Google Scholar] [CrossRef]
- Goran, M.I.; Dumke, K.; Bouret, S.G.; Kayser, B.; Walker, R.W.; Blumberg, B. The obesogenic effect of high fructose exposure during early development. Nat. Rev. Endocrinol. 2013, 9, 494–500. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and cardiometabolic health: What the evidence from sugar-sweetened beverages tells us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu. Rev. Med. 2012, 63, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- White, J.S. Challenging the fructose hypothesis: New perspectives on fructose consumption and metabolism. Adv. Nutr. 2013, 4, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. Examining the health effects of fructose. JAMA 2013, 310, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Almiron-Roig, E.; Palla, L.; Guest, K.; Ricchiuti, C.; Vint, N.; Jebb, S.A.; Drewnowski, A. Factors that determine energy compensation: A systematic review of preload studies. Nutr. Rev. 2013, 71, 458–473. [Google Scholar] [CrossRef] [PubMed]
- De Castro, J.M. The effects of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol. Behav. 1993, 53, 1133–1144. [Google Scholar] [CrossRef]
- Rolls, B.J.; Kim, S.; Fedoroff, I.C. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol. Behav. 1990, 48, 19–26. [Google Scholar] [CrossRef]
- Thomas, D.; Elliott, E.J.; Baur, L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst. Rev. 2007, CD005105. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef]
- Harding, A.-H.; Wareham, N.J.; Bingham, S.A.; Khaw, K.; Luben, R.; Welch, A.; Forouhi, N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: The European prospective investigation of cancer–Norfolk prospective study. Arch. Intern. Med. 2008, 168, 1493–1499. [Google Scholar] [CrossRef]
- Wolever, T.; Nguyen, P.-M.; Chiasson, J.-L.; Hunt, J.A.; Josse, R.G.; Palmason, C.; Rodger, N.W.; Ross, S.A.; Ryan, E.A.; Tan, M.H. Determinants of diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1994, 59, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Boeing, H.; Drogan, D.; Schulze, M.; Feskens, E.; Amiano, P.; Barricarte, A.; Clavel-Chapelon, F.; de Lauzon-Guillain, B.; Fagherazzi, G. Consumption of fatty foods and incident type 2 diabetes in populations from eight European countries. Eur. J. Clin. Nutr. 2015, 69, 455–461. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Dairy products and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Am. J. Clin. Nutr. 2013, 98, 1066–1083. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Rangan, A.; Flood, V.M.; Louie, J.C. Dietary intake and food sources of added sugar in the Australian population. Br. J. Nutr. 2016, 115, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Dodd, K.W.; Kipnis, V.; Thompson, F.E.; Potischman, N.; Schoeller, D.A.; Baer, D.J.; Midthune, D.; Troiano, R.P.; Bowles, H. Comparison of self-reported dietary intakes from the Automated Self-Administered 24-h recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. Am. J. Clin. Nutr. 2018, 107, 80–93. [Google Scholar] [CrossRef]


| Tertiles of Added Sugar | Tertiles of Total Sugar | Tertiles of Glycemic Index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (T1) | Moderate (T2) | High (T3) | Ptrend | Low (T1) | Moderate (T2) | High (T3) | Ptrend | Low (T1) | Moderate (T2) | High (T3) | Ptrend | |
| Intake (g/d) or GI a | 5 (4; 6) | 15 (14; 16) | 33 (33; 35) | 47 (46; 48) | 70 (69; 71) | 99 (97; 100) | 48 (46; 49) | 55 (53; 56) | 62 (60; 63) | |||
| Fasting insulin (pmol/L) | ||||||||||||
| Model A | 8.60 (8.20; 9.01) | 8.97 (8.57; 9.38) | 9.17 (8.77; 9.58) | 0.002 | 9.21 (8.83; 9.61) | 8.98 (8.59; 9.37) | 8.45 (8.00; 8.89) | 0.009 | 8.63 (8.19; 9.10) | 8.73 (8.29; 9.17) | 9.29 (8.87; 9.70) | 0.010 |
| Model B | 8.86 (7.99; 9.74) | 8.85 (7.98; 9.72) | 8.80 (7.92; 9.67) | 0.746 | 9.05 (8.17; 9.94) | 8.97 (8.08; 9.86) | 8.39 (7.47; 9.30) | 0.046 | 8.52 (7.93; 9.12) | 8.65 (8.07; 9.22) | 9.32 (8.73; 9.92) | 0.003 |
| HbA1c (mmol/mol) | ||||||||||||
| Model A | 36.4 (35.7; 37.1) | 36.5 (35.8; 37.1) | 36.9 (36.2; 37.6) | 0.019 | 36.8 (36.2; 37.4) | 36.4 (35.8; 37.0) | 36.6 (36.0; 37.2) | 0.155 | 36.1 (35.1; 37.1) | 36.8 (35.8; 37.8) | 36.9 (35.9; 37.9) | <0.001 |
| Model B | 36.5 (35.9; 37.0) | 36.4 (36.2; 37.0) | 36.8 (36.2; 37.3) | 0.218 | 36.7 (36.2; 37.2) | 36.4 (35.9; 36.9) | 36.7 (36.1; 37.2) | 0.566 | 36.1 (35.3; 36.9) | 36.8 (36.0; 37.6) | 36.9 (36.0; 37.7) | <0.001 |
| Fasting glucose (mmol/L) | ||||||||||||
| Model A | 6.02 (5.87; 6.18) | 6.01 (5.86; 6.16) | 6.01 (5.86; 6.16) | 0.954 | 6.10 (5.94; 6.25) | 5.96 (5.81; 6.11) | 5.97 (5.82; 6.12) | <0.001 | 5.96 (5.84; 6.09) | 6.01 (5.93; 6.18) | 6.03 (5.90; 6.15) | 0.209 |
| Model B | 6.03 (5.85; 6.21) | 6.01 (5.83; 6.18) | 5.99 (5.82; 6.17) | 0.527 | 6.07 (5.89; 6.26) | 5.96 (5.78; 6.14) | 5.99 (5.80; 6.17) | 0.024 | 5.96 (5.82; 6.12) | 6.04 (5.89; 6.19) | 6.03 (5.87; 6.18) | 0.131 |
| C-peptide (pmol/L) | ||||||||||||
| Model A | 719 (650; 788) | 731 (663; 801) | 744 (674; 813) | 0.001 | 746 (681; 811) | 735 (670; 800) | 706 (640; 772) | 0.038 | 703 (615; 791) | 741 (654; 829) | 751 (663; 838) | 0.006 |
| Model B | 736 (713; 759) | 720 (699; 742) | 717 (694; 740) | 0.909 | 738 (717; 760) | 732 (711; 754) | 698 (673; 722) | 0.045 | 702 (665; 740) | 736 (700; 771) | 740 (703; 777) | 0.005 |
| Tertiles of Added Sugar | Tertiles of Total Sugar | Tertiles of Glycemic Index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (T1) | Moderate (T2) | High (T3) | Ptrend | Low (T1) | Moderate (T2) | High (T3) | Ptrend | Low (T1) | Moderate (T2) | High (T3) | Ptrend | |
| Intake (g/d) or GI a | 5 (4; 6) | 15 (14; 16) | 33 (33; 35) | 47 (46; 48) | 70 (69; 71) | 99 (98; 100) | 48 (46; 49) | 55 (53; 56) | 62 (60; 63) | |||
| Body fat (%) | ||||||||||||
| Model A | 35.7 (29.2; 42.1) | 38.0 (31.6; 44.4) | 38.7 (32.3; 45.1) | <0.001 | 36.5 (30.7; 42.4) | 37.3 (31.5; 43.2) | 38.5 (32.7; 44.4) | <0.001 | 37.0 (30.8; 43.2) | 37.3 (31.1; 43.5) | 37.8 (31.6; 44.0) | 0.568 |
| Model B | 36.2 (29.6; 42.7) | 37.7 (31.2; 44.3) | 38.5 (31.9; 45.0) | <0.001 | 36.3 (29.8; 42.7) | 37.3 (30.8; 43.7) | 39.2 (32.7; 45.6) | <0.001 | 37.6 (31.2; 43.9) | 37.4 (31.1; 43.7) | 37.3 (31.0; 43.7) | 0.656 |
| BMI | ||||||||||||
| Model A | 30.0 (27.8; 32.2) | 30.8 (28.6; 33.0) | 31.0 (28.8; 33.2) | <0.0001 | 30.7 (28.7; 32.7) | 30.6 (28.6; 32.6) | 30.4 (28.4; 32.3) | 0.278 | 29.7 (27.0; 32.5) | 30.6 (27.9; 33.4) | 31.4 (28.7; 34.1) | 0.022 |
| Model B | 30.1 (27.7; 32.5) | 30.8 (28.4; 33.2) | 30.9 (28.5; 33.3) | 0.006 | 30.4 (28.1; 32.8) | 30.6 (28.3; 33.0) | 30.8 (28.4; 33.1) | 0.233 | 29.8 (26.9; 32.7) | 30.6 (27.8; 33.5) | 31.4 (28.5; 34.3) | 0.142 |
| Waist circumference (cm) | ||||||||||||
| Model A | 102.1 (96.6; 107.5) | 102.5 (97.0; 107.9) | 103.5 (98.1; 108.9) | 0.024 | 103.8 (98.8; 109.0) | 102.4 (97.3; 107.5) | 101.5 (96.4; 106.6) | <0.001 | 100.1 (92.3; 107.8) | 102.7 (94.9; 110.4) | 105.5 (97.8; 113.3) | <0.0001 |
| Model B | 102.3 (96.2; 108.4) | 102.7 (96.6; 108.9) | 103.3 (97.2; 109.4) | 0.188 | 103.2 (97.3; 109.2) | 102.6 (96.6; 108.6) | 102.4 (96.4; 108.4) | 0.220 | 100.1 (91.9; 108.2) | 102.6 (94.5; 110.7) | 105.7 (97.6; 113.8) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, K.; Jalo, E.; Kaartinen, N.E.; Simpson, L.; Taylor, M.A.; Muirhead, R.; Raben, A.; Macdonald, I.A.; Fogelholm, M.; Brand-Miller, J. Longitudinal Associations of Dietary Sugars and Glycaemic Index with Indices of Glucose Metabolism and Body Fatness during 3-Year Weight Loss Maintenance: A PREVIEW Sub-Study. Nutrients 2023, 15, 2083. https://doi.org/10.3390/nu15092083
Della Corte K, Jalo E, Kaartinen NE, Simpson L, Taylor MA, Muirhead R, Raben A, Macdonald IA, Fogelholm M, Brand-Miller J. Longitudinal Associations of Dietary Sugars and Glycaemic Index with Indices of Glucose Metabolism and Body Fatness during 3-Year Weight Loss Maintenance: A PREVIEW Sub-Study. Nutrients. 2023; 15(9):2083. https://doi.org/10.3390/nu15092083
Chicago/Turabian StyleDella Corte, Karen, Elli Jalo, Niina E. Kaartinen, Liz Simpson, Moira A. Taylor, Roslyn Muirhead, Anne Raben, Ian A. Macdonald, Mikael Fogelholm, and Jennie Brand-Miller. 2023. "Longitudinal Associations of Dietary Sugars and Glycaemic Index with Indices of Glucose Metabolism and Body Fatness during 3-Year Weight Loss Maintenance: A PREVIEW Sub-Study" Nutrients 15, no. 9: 2083. https://doi.org/10.3390/nu15092083