Pancreatic Ductal Adenocarcinoma and Nutrition: Exploring the Role of Diet and Gut Health
Abstract
:1. Introduction
2. Materials and Methods
3. PDAC Immunology
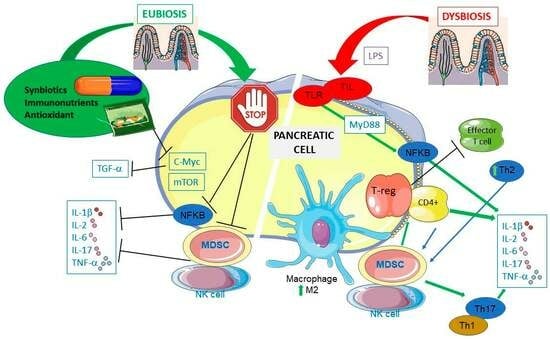
4. PDAC and Gut Microbiota
5. Role of Nutrition and PDAC
5.1. Immunonutrients and PDAC
5.2. Antioxidants and PDAC
5.3. Diet, Gut Microbiota and PDAC
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodríguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A Faecal Microbiota Signature with High Specificity for Pancreatic Cancer. Gut 2022, 71, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Département Prévention Cancer Environnement, Centre Léon Bérard Cancer Du Pancréas et Facteurs de Risque. Available online: https://www.cancer-environnement.fr/fiches/cancers/cancer-du-pancreas (accessed on 5 June 2023).
- Ushio, J.; Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; et al. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics 2021, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Data Visualization Tools for Exploring the Global Cancer Burden in 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Ertz-Archambault, N.; Keim, P.; Von Hoff, D. Microbiome and Pancreatic Cancer: A Comprehensive Topic Review of Literature. World J. Gastroenterol. 2017, 23, 1899. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851. [Google Scholar] [CrossRef]
- Lee, S. Société canadienne du cancer Antigène Carbohydrate 19-9 (CA 19-9). Available online: https://cancer.ca/fr/treatments/tests-and-procedures/carbohydrate-antigen-19-9-ca-19-9 (accessed on 5 June 2023).
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Kanda, M.; Sadakari, Y.; Borges, M.; Topazian, M.; Farrell, J.; Syngal, S.; Lee, J.; Kamel, I.; Lennon, A.M.; Knight, S.; et al. Mutant TP53 in Duodenal Samples of Pancreatic Juice From Patients With Pancreatic Cancer or High-Grade Dysplasia. Clin. Gastroenterol. Hepatol. 2013, 11, 719–730.e5. [Google Scholar] [CrossRef]
- Princivalle, A.; Monasta, L.; Butturini, G.; Bassi, C.; Perbellini, L. Pancreatic Ductal Adenocarcinoma Can Be Detected by Analysis of Volatile Organic Compounds (VOCs) in Alveolar Air. BMC Cancer 2018, 18, 529. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic Cancer: A Review of Clinical Diagnosis, Epidemiology, Treatment and Outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Salem, A.A.; Mackenzie, G.G. Pancreatic Cancer: A Critical Review of Dietary Risk. Nutr. Res. 2018, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Olakowski, M.; Bułdak, Ł. Modifiable and Non-Modifiable Risk Factors for the Development of Non-Hereditary Pancreatic Cancer. Medicina 2022, 58, 978. [Google Scholar] [CrossRef] [PubMed]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Sweetened Beverages Consumption and Pancreatic Cancer: A Meta-Analysis. Nutr. Cancer 2019, 71, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Wirkus, J.; Ead, A.S.; Mackenzie, G.G. Impact of Dietary Fat Composition and Quantity in Pancreatic Carcinogenesis: Recent Advances and Controversies. Nutr. Res. 2021, 88, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Nardi, M.; Cinnirella, A.; Campagnoli, E.; Maffeo, M.; Perrone, P.M.; Shishmintseva, V.; Grosso, F.M.; Castrofino, A.; Castaldi, S.; et al. Adherence to Mediterranean Diet and Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2403. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Rio, P.; Massaro, M.G.; Caldarelli, M.; Frank, G.; Della-Morte, D.; Gasbarrini, A.; Gambassi, G.; De Lorenzo, A.; et al. Role of Nutrients in Modulating Microbiota and Immunity in COVID-19 Disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5927–5945. [Google Scholar] [CrossRef]
- Merra, G.; Gualtieri, P.; De Lorenzo, A.; Capacci, A.; Frank, G.; Dri, M.; Di Renzo, L.; Marchetti, M. Impact Of Precision Nutrition On Microbiota And Obesity. Curr. Nutr. Food Sci. 2023, 19. E-pub Ahead of Print. [Google Scholar]
- Memba, R.; Duggan, S.N.; Ni Chonchubhair, H.M.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.M.; et al. The Potential Role of Gut Microbiota in Pancreatic Disease: A Systematic Review. Pancreatology 2017, 17, 867–874. [Google Scholar] [CrossRef]
- The University of Melbourne. Which Review Is That? A Guide to Review Types. Review of Reviews. Available online: https://unimelb.libguides.com/c.php?g=933440&p=6911511 (accessed on 9 September 2023).
- Grey, A.; Bolland, M.J.; Avenell, A.; Klein, A.A.; Gunsalus, C.K. Check for Publication Integrity before Misconduct. Nature 2020, 577, 167–169. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, D.; Saviano, A.; Newton, E.E.; Serricchio, M.L.; Dal Lago, A.A.; Gasbarrini, A.; Cianci, R. Gut Microbiota-Immune System Crosstalk and Pancreatic Disorders. Mediat. Inflamm. 2018, 2018, 7946431. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef] [PubMed]
- di Magliano, M.P.; Logsdon, C.D. Roles for KRAS in Pancreatic Tumor Development and Progression. Gastroenterology 2013, 144, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Cianci, R.; Pagliari, D.; Pietroni, V.; Landolfi, R.; Pandolfi, F. Tissue Infiltrating Lymphocytes: The Role of Cytokines in Their Growth and Differentiation. J. Biol. Regul. Homeost. Agents 2010, 24, 239–249. [Google Scholar]
- Cianci, R.; Franza, L.; Schinzari, G.; Rossi, E.; Ianiro, G.; Tortora, G.; Gasbarrini, A.; Gambassi, G.; Cammarota, G. The Interplay between Immunity and Microbiota at Intestinal Immunological Niche: The Case of Cancer. Int. J. Mol. Sci. 2019, 20, 501. [Google Scholar] [CrossRef]
- Inman, K.S. Complex Role for the Immune System in Initiation and Progression of Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 11160. [Google Scholar] [CrossRef]
- Orlacchio, A.; Mazzone, P. The Role of Toll-like Receptors (TLRs) Mediated Inflammation in Pancreatic Cancer Pathophysiology. Int. J. Mol. Sci. 2021, 22, 12743. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Dong, S.; Xu, H.; Zhou, W. Association of the Microbiota and Pancreatic Cancer: Opportunities and Limitations. Front. Immunol. 2022, 13, 844401. [Google Scholar] [CrossRef]
- Rolfo, C.; Giovannetti, E.; Martinez, P.; McCue, S.; Naing, A. Applications and Clinical Trial Landscape Using Toll-like Receptor Agonists to Reduce the Toll of Cancer. Npj Precis. Oncol. 2023, 7, 26. [Google Scholar] [CrossRef]
- Evans, A.; Costello, E. The Role of Inflammatory Cells in Fostering Pancreatic Cancer Cell Growth and Invasion. Front. Physio. 2012, 3, 270. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, H.; Mou, Y.; Li, L.; Jin, W. Functions and Clinical Applications of Exosomes in Pancreatic Cancer. Mol. Biol. Rep. 2022, 49, 11037–11048. [Google Scholar] [CrossRef] [PubMed]
- Pergamo, M.; Miller, G. Myeloid-Derived Suppressor Cells and Their Role in Pancreatic Cancer. Cancer Gene Ther. 2017, 24, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yako, Y.Y.; Kruger, D.; Smith, M.; Brand, M. Cytokines as Biomarkers of Pancreatic Ductal Adenocarcinoma: A Systematic Review. PLoS ONE 2016, 11, e0154016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, H.; Zhang, S.; Wei, Y.; Liu, S. The Interplay Between Inflammation and Stromal Components in Pancreatic Cancer. Front. Immunol. 2022, 13, 850093. [Google Scholar] [CrossRef] [PubMed]
- Myo Min, K.K.; Ffrench, C.B.; Jessup, C.F.; Shepherdson, M.; Barreto, S.G.; Bonder, C.S. Overcoming the Fibrotic Fortress in Pancreatic Ductal Adenocarcinoma: Challenges and Opportunities. Cancers 2023, 15, 2354. [Google Scholar] [CrossRef]
- Falcomatà, C.; Bärthel, S.; Schneider, G.; Rad, R.; Schmidt-Supprian, M.; Saur, D. Context-Specific Determinants of the Immunosuppressive Tumor Microenvironment in Pancreatic Cancer. Cancer Discov. 2023, 13, 278–297. [Google Scholar] [CrossRef]
- Hawa, Z.; Haque, I.; Ghosh, A.; Banerjee, S.; Harris, L.; Banerjee, S. The miRacle in Pancreatic Cancer by miRNAs: Tiny Angels or Devils in Disease Progression. Int. J. Mol. Sci. 2016, 17, 809. [Google Scholar] [CrossRef]
- Sato, H.; Sasaki, K.; Hara, T.; Tsuji, Y.; Arao, Y.; Otsuka, C.; Hamano, Y.; Ogita, M.; Kobayashi, S.; di Luccio, E.; et al. Pancreatic Cancer Research beyond DNA Mutations. Biomolecules 2022, 12, 1503. [Google Scholar] [CrossRef]
- Panthangi, V.; Cyril Kurupp, A.R.; Raju, A.; Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.D.; Zafar, S.; Khan, S. Association Between Helicobacter Pylori Infection and the Risk of Pancreatic Cancer: A Systematic Review Based on Observational Studies. Cureus 2022, 14, e28543. [Google Scholar] [CrossRef]
- Pfisterer, N.; Lingens, C.; Heuer, C.; Dang, L.; Neesse, A.; Ammer-Herrmenau, C. The Microbiome in PDAC—Vantage Point for Future Therapies? Cancers 2022, 14, 5974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Liu, S.; Yang, M. Clinical Diagnosis and Management of Pancreatic Cancer: Markers, Molecular Mechanisms, and Treatment Options. World J. Gastroenterol. 2022, 28, 6827–6845. [Google Scholar] [CrossRef] [PubMed]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; McKinley, K.L.; Triplett, E.W.; Hughes, S.J.; Trevino, J.G. The Oral Microbiome, Pancreatic Cancer and Human Diversity in the Age of Precision Medicine. Microbiome 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Sobocki, B.K.; Kaźmierczak-Siedlecka, K.; Folwarski, M.; Hawryłkowicz, V.; Makarewicz, W.; Stachowska, E. Pancreatic Cancer and Gut Microbiome-Related Aspects: A Comprehensive Review and Dietary Recommendations. Nutrients 2021, 13, 4425. [Google Scholar] [CrossRef]
- Emanuel, A.; Krampitz, J.; Rosenberger, F.; Kind, S.; Rötzer, I. Nutritional Interventions in Pancreatic Cancer: A Systematic Review. Cancers 2022, 14, 2212. [Google Scholar] [CrossRef]
- Cañamares-Orbís, P.; García-Rayado, G.; Alfaro-Almajano, E. Nutritional Support in Pancreatic Diseases. Nutrients 2022, 14, 4570. [Google Scholar] [CrossRef] [PubMed]
- Kasvis, P.; Kilgour, R.D. Diet and Exercise Interventions in Patients With Pancreatic Cancer: A Scoping Review. Pancreas 2021, 50, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Cortez, N.E.; Mackenzie, G.G. Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives. Nutrients 2021, 13, 3202. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary Fiber and Health Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef]
- Guo, Z.; Hong, Y.; Cheng, Y. Dietary Inflammatory Index and Pancreatic Cancer Risk: A Systematic Review and Dose–Response Meta-Analysis. Public Health Nutr. 2021, 24, 6427–6435. [Google Scholar] [CrossRef]
- Jayedi, A.; Emadi, A.; Shab-Bidar, S. Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose-Response Meta-Analysis. Adv. Nutr. 2018, 9, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Han, Y.M.; An, J.M.; Kang, E.A.; Park, Y.J.; Cha, J.Y.; Hahm, K.B. Role of Omega-3 Polyunsaturated Fatty Acids in Preventing Gastrointestinal Cancers: Current Status and Future Perspectives. Expert Rev. Anticancer. Ther. 2018, 18, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Sun, X.; Lu, S.; Liu, S. Vitamin Intake and Pancreatic Cancer Risk Reduction: A Meta-Analysis of Observational Studies. Medicine 2018, 97, e0114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yung, K.K.-L.; Ko, J.K.-S. Therapeutic Intervention in Cancer by Isoliquiritigenin from Licorice: A Natural Antioxidant and Redox Regulator. Antioxidants 2022, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Tazehkand, A.P.; Soofiyani, S.R.; Hosseini, K.; Martorell, M.; Tarhriz, V.; Ahangari, H.; Cruz-Martins, N.; Sharifi-Rad, J.; Almarhoon, Z.M.; et al. Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects. Oxidative Med. Cell. Longev. 2021, 2021, 4393266. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Fliegel, L. Role of Silymarin in Cancer Treatment: Facts, Hypotheses, and Questions. J. Evid. Based Complement. Altern. Med. 2022, 27, 2515690X2110688. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xiao, Y.; Sun, J.; Ji, B.; Luo, S.; Wu, B.; Zheng, C.; Wang, P.; Xu, F.; Cheng, K.; et al. New Possible Silver Lining for Pancreatic Cancer Therapy: Hydrogen Sulfide and Its Donors. Acta Pharm. Sin. B 2021, 11, 1148–1157. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Zhu, Y. Potential Roles of the Gut Microbiota in Pancreatic Carcinogenesis and Therapeutics. Front. Cell. Infect. Microbiol. 2022, 12, 872019. [Google Scholar] [CrossRef]
- Ibrahim, M.O.; Abuhijleh, H.; Tayyem, R. What Dietary Patterns and Nutrients Are Associated with Pancreatic Cancer? Literature Review. Cancer Manag. Res. 2023, 15, 17–30. [Google Scholar] [CrossRef]

| Author | Title | Type of Paper | Date | Finding |
|---|---|---|---|---|
| Pagliari D. et al. [26] | Gut Microbiota-Immune System Crosstalk and Pancreatic Disorders | Review | 2018 | Chronic inflammation-related PDAC can arise independently of bacteria, driven by sterile inflammation triggered by intestinal dysbiosis and immune system activation through TLRs. The gut microbiota and antibiotics may affect chemotherapy responses and the tumor microenvironment, suggesting their role in treatment efficacy in PDAC. |
| Padoan A. et al. [27] | Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity | Review | 2019 | PDAC risk is heightened by inflammation and ‘metaflammation’, yet PDAC triggers an immunosuppressive inflammatory response. The interplay between cytokines and chemokines produced by inflammatory cells and those produced by cancer plays a crucial role on both cancer development and inflammatory response. The key role of TNFα is highlighted. |
| di Magliano M.P. et al. [28] | Roles for KRAS in pancreatic tumor development and progression | Review | 2013 | Sustained KRAS activity is crucial for pancreatic tumorigenesis, and whether oncogenic KRAS expression alone generates the required activation levels or if additional upstream signals are necessary remains unclear. This uncertainty suggests potential avenues for pancreatic cancer prevention, which could involve minimizing factors that activate KRAS, such as addressing inflammation and lifestyle factors. |
| Cianci R. et al. [29] | Tissue infiltrating lymphocytes: the role of cytokines in their growth and differentiation | Review | 2010 | This review focuses on the role of local tissue cytokines in influencing T-cell proliferation and differentiation, highlighting the remarkable plasticity of CD4+ T-cells with numerous differentiation possibilities. The role of TILs and their microenvironment is discussed in various diseases, including tumors, along with an exploration of the role of apoptosis and the mucosal immune environment. |
| Cianci R. et al. [30] | The Interplay between Immunity and Microbiota at Intestinal Immunological Niche: The Case of Cancer | Review | 2019 | The microbiota exerts both immune-modulated and direct effects on the carcinogenesis of the gastrointestinal tract, including organs like the pancreas, not directly colonized by microbes. It plays a key role in cancer development, therapy response, and even the influences therapeutic strategies, highlighting the potential benefits of the gut microbiota modulation for both cancer patients and as a preventive approach for the general population. |
| Inman K.S. et al. [31] | Complex role for the immune system in initiation and progression of pancreatic cancer | Review | 2014 | In PDAC, there is a notable presence of various immunosuppressive cell types and a dysfunction of the immune response. This immune dysfunction involves the activation of immunosuppressive cells, the presence of immune cells that support tumor growth, and a deficiency in functional immune cells, ultimately reducing a vital barrier to tumor growth. |
| Orlacchio A. et al. [32] | The Role of Toll-like Receptors (TLRs) Mediated Inflammation in Pancreatic Cancer Pathophysiology | Review | 2021 | TLRs are pivotal in PDAC as they trigger both pro-inflammatory pathways that create a favorable tumor microenvironment and pathways leading to the production of immunosuppressive cytokines. Utilizing TLR agonists and antagonists in cancer therapy holds promise for enhancing survival rates. |
| Chen Z. et al. [33] | Association of the Microbiota and Pancreatic Cancer: Opportunities and Limitations | Review | 2022 | The interaction between pancreatic cancer and the microbiota involves both tumor-promoting and antitumor effects, showing changes in the “immune microsystem” within the tumor microenvironment. However, the great diversity of the microbiota and its multiple interactions with human factors such as age, sex, immune function, diet, climate, and geography pose significant challenges to harnessing the microbiota as an accurate treatment. |
| Rolfo C. et al. [34] | Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer | Review | 2023 | TLR agonists are promising for cancer treatment, particularly TLR7 and TLR9 agonists, either as a monotherapy or in combination with immune checkpoint inhibitors. While the most common side effects include several symptoms, these investigational TLR agonists, like CV8102 and tilsotolimod, do not worsen the toxicity of immune checkpoint inhibitors in combination regimens, which sets them apart from the discontinued TLR agonists with increased risk of adverse events. |
| Evans A. et al. [35] | The role of inflammatory cells in fostering pancreatic cancer cell growth and invasion | Mini Review | 2012 | Tumors can evade immune surveillance. The transition from an antitumor immune response to immune tolerance in the development of intraductal papillary mucinous neoplasm suggests the tumor-promoting roles of inflammatory cells, including immune suppression, angiogenesis, and metastasis, and highlights the need for further research to fully understand these roles in PDAC. |
| Jiang Z. et al. [36] | Functions and clinical applications of exosomes in pancreatic cancer | Review | 2022 | The use of exosomes as biomarkers for pancreatic cancer and their potential applications in cancer treatment are discussed, offering insights into the development of diagnostic tools and treatment strategies. However, challenges such as technical complexities, early stage clinical trials, and insufficient mechanistic research need to be addressed for effective clinical implementation. |
| Pergamo M. et al. [37] | Myeloid-derived suppressor cells and their role in pancreatic cancer | Review | 2017 | MDSCs play a crucial role in balancing immunogenic and tolerogenic signals by employing various mechanisms of immunosuppression. While targeting MDSCs has shown limited success to date, they represent a potential avenue for new immunotherapies that may prove effective in combating pancreatic cancer. |
| Yako Y.Y. et al. [38] | Cytokines as biomarkers of pancreatic ductal adenocarcinoma: a systematic review | Systematic Review | 2016 | Several studies consistently reported increased concentrations of IL-1β, IL-6, IL-8, VEGF, TGF, and IL-10 in PDAC patients, but their diagnostic performance has not been extensively tested, and they require validation in different study populations. These cytokines were associated with the severity of PDAC, suggesting a potential role as prognostic biomarkers, but further clinical evaluations are necessary to establish their clinical value for diagnostic, prognostic, or predictive purposes. |
| Li Y. et al. [39] | The Interplay Between Inflammation and Stromal Components in Pancreatic Cancer | Review | 2022 | The interaction between pancreatic cancer cells, stromal cells, and cytokines creates an inflammatory and immunosuppressive microenvironment, influencing various aspects of pancreatic cancer. Manipulating cytokine pathways holds promise for pancreatic cancer treatment, but challenges exist in translating animal model findings to human cancer. Single-cell sequencing technologies are aiding in defining the diversity and precise roles of stromal components in the tumor microenvironment, potentially revolutionizing personalized therapeutic approaches in the future. |
| Myo Min K.K. et al. [40] | Overcoming the Fibrotic Fortress in Pancreatic Ductal Adenocarcinoma: Challenges and Opportunities | Review | 2023 | PDAC presents a persistently low 5-year survival rate and limited benefits from conventional cancer treatments, including immunotherapy. A major challenge in PDAC therapy is effectively delivering drugs to the tumor due to the dense and complex tumor microenvironment, but renewed efforts in understanding and targeting this microenvironment offer hope for improved treatment efficacy. |
| Falcomatà C. et al. [41] | Context-Specific Determinants of the Immunosuppressive Tumor Microenvironment in Pancreatic Cancer | Review | 2023 | PDAC exhibits significant diversity, but a common feature is immunosuppression, limiting the effectiveness of immunotherapies. Understanding the distinct immunosuppressive niches and mechanisms in different PDAC subtypes is crucial, and advances in mouse modeling and high-throughput technologies can provide insights for the development of combinatorial immunomodulatory therapies to overcome these barriers. |
| Hawa Z. et al. [42] | The miRacle in Pancreatic Cancer by miRNAs: Tiny Angels or Devils in Disease Progression | Review | 2016 | MicroRNAs play a vital role in regulating gene expression, impacting various aspects of pancreatic cancer progression. These miRNAs have diagnostic and prognostic potential and could be considered as therapeutic tools, though addressing carrier-induced toxicity and further understanding that their downstream targets are crucial for their effective clinical application in combating pancreatic cancer. |
| Sato H. et al. [43] | Pancreatic Cancer Research beyond DNA Mutations | Review | 2022 | The early diagnosis of PDAC is crucial for improving patient survival. Understanding intercellular communication within the tumor microenvironment at the single-cell level may pave the way for highly individualized therapies, offering innovative treatments for advanced stages of the disease. Additionally, emerging diagnostic methods like VOC analysis hold potential for enhancing PDAC monitoring and may provide insights beyond DNA mutations, offering new avenues for addressing challenging cancers like PDAC. |
| Author | Title | Type of Paper | Date | Finding |
|---|---|---|---|---|
| Ertz-Archambault N. et al. [7] | Microbiome and pancreatic cancer: a comprehensive topic review of literature | Review | 2017 | The connection between dysbiosis and pancreatic cancer, a disease known for its poor prognosis due to its late detection and resistance to treatment, is controversial. Mouse models indicate that altering the commensal microbiome can influence how tumors respond to chemotherapy in different cancer types, showing potential for improved survival and reduced cachexia in PDAC patients, offering insights into early screening biomarkers and novel therapeutic approaches. |
| Panthangi V. et al. [44] | Association Between Helicobacter pylori Infection and the Risk of Pancreatic Cancer: A Systematic Review Based on Observational Studies | Systematic Reviews and Meta-Analysis | 2022 | Few studies demonstrated a significant association between H. pylori infection and pancreatic cancer risk, primarily within European and Asian populations, with only one involving North Americans, indicating a weak association that does not provide conclusive evidence of H. pylori’s role in pancreatic cancer. |
| Pfisterer N. et al. [45] | The Microbiome in PDAC-Vantage Point for Future Therapies? | Review | 2022 | PDAC exhibits its own unique microbiome, influencing cancer development, treatment response, and patient prognosis. While the microbiome presents potential as a diagnostic and therapeutic tool in PDAC, inconsistencies in microbial composition require stringent decontamination procedures. Promising approaches, such as bacteriophages and fecal microbiome transplants, are still largely theoretical, and clinical trials are exploring the microbiome’s role as a biomarker for prognosis and surgical outcomes in PDAC. |
| Zhang C.Y. et al. [46] | Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options | Review | 2022 | Despite the approval of some therapies, overall survival rates remain poor, highlighting the need for novel immunotherapies, combined treatments, and advanced delivery methods to improve outcomes and combat drug resistance in PDAC patients, underscoring the importance of further clinical trials to evaluate these approaches. |
| Herremans, K.M. et al. [47] | The oral microbiome, pancreatic cancer and human diversity in the age of precision medicine | Review | 2022 | The oral microbiome, which changes in pancreatic cancer patients even before disease onset, can potentially serve as a noninvasive screening method to identify those at higher risk of developing cancer. Additionally, analysis of the oral microbiome could help guide treatment choices for patients and offer potential therapeutic options through microbial modification. |
| Sobocki B.K. et al. [48] | Pancreatic cancer and gut microbiome-related aspects: a comprehensive review and dietary recommendations | Review | 2021 | The connection between the gut microbiota and pancreatic cancer reveals that alterations in the gut microbiota can influence the development of pancreatic cancer. Methods like prebiotics, probiotics, next-generation probiotics, synbiotics, and fecal microbiota transplantation have the potential to be used as therapeutic strategies for pancreatic cancer. |
| Author | Title | Type of Paper | Date | Finding |
|---|---|---|---|---|
| Emanuel A. et al. [49] | Nutritional Interventions in Pancreatic Cancer: A Systematic Review | Systematic Review | 2022 | The nutritional management of cachexia, malnutrition, and weight loss in pancreatic cancer is investigated. The findings suggest that enteral nutrition can have positive effects on various aspects, while dietary supplements enriched with omega-3 fatty acids appear to help maintain or increase body weight and lean body mass. |
| Cañamares-Orbís P. et al. [50] | Nutritional Support in Pancreatic Diseases | Review | 2022 | Patients with pancreatic diseases often face malnutrition, which is significant in chronic pancreatitis. Early diagnosis is key, as malnutrition can be due to both systemic factors related to the disease and specific factors like pancreatic enzyme insufficiency. Nutritional assessments, PERT, and personalized nutrition strategies are crucial in managing these conditions, and nutritional support can improve life quality and treatment tolerance, especially in pancreatic cancer. |
| Kasvis P. et al. [51] | Diet and Exercise Interventions in Patients With Pancreatic Cancer: A Scoping Review | Review | 2021 | This review evaluates the research gaps in dietary and/or exercise interventions previously studied in outpatients with pancreatic cancer. |
| Cortez N.E. et al. [52] | Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives | Review | 2021 | This review highlights the impact of the ketogenic diet (KD) in PDAC treatment and cachexia, reporting the potential anticancer and anti-cachexia effects of KD. |
| Veronese N. et al. [53] | Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses | Umbrella Review | 2018 | A comprehensive overview on the associations between dietary fiber intake and inflammation, cancer and cardiovascular disease. |
| Guo Z. et al. [54] | Dietary inflammatory index and pancreatic cancer risk: a systematic review and dose–response meta-analysis | Systematic Review and Meta-Analysis | 2021 | The review reports the link between pancreatic cancer risk and the Dietary Inflammatory Index (DII) score, which assesses the inflammatory component of foods. A high DII score corresponds to eating habits with high inflammatory characteristics. |
| Jayedi A. et al. [55] | Dietary Inflammatory Index and Site-Specific Cancer Risk: A Systematic Review and Dose–response Meta-Analysis | Review | 2018 | This review reports the associations of the Dietary Inflammatory Index (DII) and site-specific cancer risk. |
| Lee H.J. et al. [56] | Role of omega-3 polyunsaturated fatty acids in preventing gastrointestinal cancers: current status and future perspectives | Review | 2018 | Potent natural products like n-3 PUFAs, known for their cancer-preventive properties, have shown potential in altering cancer cell properties and the tumor microenvironment, but more research, including clinical trials, is needed to determine their optimal use and efficacy in cancer prevention. |
| Liu Y. et al. [57] | Vitamin intake and pancreatic cancer risk reduction: A meta-analysis of observational studies | Systematic Review and Meta-Analysis | 2018 | The intake of vitamins, especially vitamin D and vitamin B12, was associated with a moderate reduction in the risk of pancreatic cancer. |
| Zhang Z. et al. [58] | Therapeutic Intervention in Cancer by Isoliquiritigenin from Licorice: A Natural Antioxidant and Redox Regulator | Review | 2022 | Chemoresistance and poor efficacy of combination therapies with drugs like gemcitabine have been significant challenges in treating pancreatic cancer. Targeting autophagy, specifically inhibiting it, may be a promising strategy to improve chemotherapy, and isoflavonoids from herbal sources like ISL could help overcome chemoresistance, potentially enhancing the survival and quality of life for patients. |
| Asgharian P. et al. [59] | Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects | Review | 2021 | This review highlights the effects of quercetin on pancreatic cancer. It inhibits c-Myc and TGF-α expression and promotes autophagy and cancer cell apoptosis |
| Koltai T. et al. [60] | Role of Silymarin in Cancer Treatment: Facts, Hypotheses, and Questions | Review | 2021 | Silymarin exhibits significant antitumoral effects, and clinical trials are needed to determine its clinical applications, particularly its dual effects. The use of high doses, as well as modified pharmaceutical forms, is recommended due to silymarin’s low absorption, and it has potential in cancer treatment and prevention. |
| Hu X. et al. [61] | New possible silver lining for pancreatic cancer therapy: Hydrogen sulfide and its donors | Review | 2021 | H2S donors have shown potential as therapeutic agents in pancreatic cancer, and the development of dual H2S and NO donor drugs is underway to address cell proliferation, apoptosis, and cell cycle modulation. However, challenges such as targeted delivery, H2S concentration maintenance, and clarification of the dual H2S/NO donor’s mechanisms need further attention. |
| Yang, Q. et al. [62] | Potential Roles of the Gut Microbiota in Pancreatic Carcinogenesis and Therapeutics. | Review | 2022 | The gut microbiota is crucial in pancreatic cancer regulation, offering insights into mechanisms, risk prediction, diagnosis, and prognosis. This research highlights the potential of probiotics in combination with chemotherapy and immunotherapy for pancreas cancer treatment, emphasizing the importance of investigating cancer-related microbiome approaches and personalized treatments to enhance patient outcomes. |
| Ibrahim, M.O. et al. [63] | What Dietary Patterns and Nutrients Are Associated with Pancreatic Cancer? Literature Review. | Review | 2023 | The high consumption of sugar, fructose, and fat may elevate pancreas cancer risk, while dietary fiber, fat-soluble vitamins, water-soluble vitamins, and minerals may have a protective effect. Additionally, dietary patterns rich in plant-based foods, antioxidants, and polyphenols appear to reduce cancer risk, while patterns high in red and processed meats, sugar, and sugar-sweetened soft drinks are associated with an increased risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtieri, P.; Cianci, R.; Frank, G.; Pizzocaro, E.; De Santis, G.L.; Giannattasio, S.; Merra, G.; Butturini, G.; De Lorenzo, A.; Di Renzo, L. Pancreatic Ductal Adenocarcinoma and Nutrition: Exploring the Role of Diet and Gut Health. Nutrients 2023, 15, 4465. https://doi.org/10.3390/nu15204465
Gualtieri P, Cianci R, Frank G, Pizzocaro E, De Santis GL, Giannattasio S, Merra G, Butturini G, De Lorenzo A, Di Renzo L. Pancreatic Ductal Adenocarcinoma and Nutrition: Exploring the Role of Diet and Gut Health. Nutrients. 2023; 15(20):4465. https://doi.org/10.3390/nu15204465
Chicago/Turabian StyleGualtieri, Paola, Rossella Cianci, Giulia Frank, Erica Pizzocaro, Gemma Lou De Santis, Silvia Giannattasio, Giuseppe Merra, Giovanni Butturini, Antonino De Lorenzo, and Laura Di Renzo. 2023. "Pancreatic Ductal Adenocarcinoma and Nutrition: Exploring the Role of Diet and Gut Health" Nutrients 15, no. 20: 4465. https://doi.org/10.3390/nu15204465