Classification and Special Nutritional Needs of SGA Infants and Neonates of Multiple Pregnancies
Abstract
:1. Introduction
2. The Distinction between Constitutional SGA and FGR Infants
3. Why Do FGR Infants Have Different Nutritional Needs?
4. The SGA Infant and Metabolic Consequences
5. Fetal Growth Restriction in Multiple Gestation
6. The Preterm FGR Infant
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Supplementary Digital Content no.1. ESPGHAN Committee of Nutrition (CoN) Position Paper on Enteral Nutrition for Preterm Infants: Introduction, Methods and Limitations. Available online: http://links.lww.com/MPG/C974 (accessed on 6 April 2023).
- Fleig, L.; Hagan, J.; Lee, M.L.; Abrams, S.A.; Hawthorne, K.M.; Hair, A.B. Growth outcomes of small for gestational age preterm infants before and after implementation of an exclusive human milk-based diet. J. Perinatol. 2021, 41, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Shah, S.R. Nutrient deficiencies in the premature infant. Pediatr. Clin. N. Am. 2009, 56, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.; Kempley, S.; Leaf, A. Feeding growth restricted preterm infants with abnormal antenatal Doppler results. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F359–F363. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Morris, J.M.; Leslie, G.I.; Kelly, P.J.; Gallery, E.D. The long-term effects of prematurity and intrauterine growth restriction on cardiovascular, renal, and metabolic function. Int. J. Pediatr. 2010, 2010, 280402. [Google Scholar] [CrossRef] [Green Version]
- Martín-Calvo, N.; Goni, L.; Tur, J.A.; Martínez, J.A. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: Systematic review and meta-analysis. Obes. Rev. 2022, 23 (Suppl. S1), e13380. [Google Scholar] [CrossRef]
- Damhuis, S.E.; Ganzevoort, W.; Gordijn, S.J. Abnormal Fetal Growth: Small for Gestational Age, Fetal Growth Restriction, Large for Gestational Age: Definitions and Epidemiology. Obstet. Gynecol. Clin. N. Am. 2021, 48, 267–279. [Google Scholar] [CrossRef]
- Maulik, D. Fetal growth restriction: The etiology. Clin. Obstet. Gynecol. 2006, 49, 228–235. [Google Scholar] [CrossRef]
- Kesavan, K.; Devaskar, S.U. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423. [Google Scholar] [CrossRef]
- WHO. Expert Committee on Physical Status: The Use Interpretation of Anthropometry & World Health Organization. In Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.A.; Prefumo, F.; Frusca, T.; Visser, G.H.A.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef]
- Lee, A.C.; Kozuki, N.; Cousens, S.; Stevens, G.A.; Blencowe, H.; Silveira, M.F.; Sania, A.; Rosen, H.E.; Schmiegelow, C.; Adair, L.S.; et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: Analysis of CHERG datasets. BMJ 2017, 358, j3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Onis, M.; Blössner, M.; Villar, J. Levels and patterns of intrauterine growth retardation in developing countries. Eur. J. Clin. Nutr. 1998, 52 (Suppl. S1), S5–S15. [Google Scholar] [PubMed]
- He, H.; Miao, H.; Liang, Z.; Zhang, Y.; Jiang, W.; Deng, Z.; Tang, J.; Liu, G.; Luo, X. Prevalence of small for gestational age infants in 21 cities in China, 2014–2019. Sci. Rep. 2021, 11, 7500. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.M.; Horbar, J.D.; Badger, G.J.; Ohlsson, A.; Golan, A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 2000, 182, 198–206. [Google Scholar] [CrossRef]
- Lemons, J.A.; Bauer, C.R.; Oh, W.; Korones, S.B.; Papile, L.A.; Stoll, B.J.; Verter, J.; Temprosa, M.; Wright, L.L.; Ehrenkranz, R.A.; et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics 2001, 107, E1. [Google Scholar] [CrossRef] [Green Version]
- Fang, S. Management of preterm infants with intrauterine growth restriction. Early Hum. Dev. 2005, 81, 889–900. [Google Scholar] [CrossRef]
- Ananth, C.V.; Vintzileos, A.M. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum. Dev. 2009, 85, 653–658. [Google Scholar] [CrossRef]
- Clausson, B.; Gardosi, J.; Francis, A.; Cnattingius, S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG 2001, 108, 830–834. [Google Scholar] [CrossRef]
- Mongelli, M.; Figueras, F.; Francis, A.; Gardosi, J. A customized birthweight centile calculator developed for an Australian population. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 128–131. [Google Scholar] [CrossRef]
- Figueras, F.; Figueras, J.; Meler, E.; Eixarch, E.; Coll, O.; Gratacos, E.; Gardosi, J.; Carbonell, X. Customised birthweight standards accurately predict perinatal morbidity. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F277–F280. [Google Scholar] [CrossRef] [Green Version]
- Gardosi, J.; Chang, A.; Kalyan, B.; Sahota, D.; Symonds, E.M. Customised antenatal growth charts. Lancet 1992, 339, 283–287. [Google Scholar] [CrossRef]
- Carberry, A.E.; Gordon, A.; Bond, D.M.; Hyett, J.; Raynes-Greenow, C.H.; Jeffery, H.E. Customised versus population-based growth charts as a screening tool for detecting small for gestational age infants in low-risk pregnant women. Cochrane Database Syst. Rev. 2014, 2014, Cd008549. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- Taine, M.; Charles, M.A.; Beltrand, J.; Rozé, J.C.; Léger, J.; Botton, J.; Heude, B. Early postnatal growth and neurodevelopment in children born moderately preterm or small for gestational age at term: A systematic review. Paediatr. Perinat. Epidemiol. 2018, 32, 268–280. [Google Scholar] [CrossRef]
- Ibáñez, L.; Lopez-Bermejo, A.; Suárez, L.; Marcos, M.V.; Díaz, M.; de Zegher, F. Visceral adiposity without overweight in children born small for gestational age. J. Clin. Endocrinol. Metab. 2008, 93, 2079–2083. [Google Scholar] [CrossRef]
- Jaquet, D.; Deghmoun, S.; Chevenne, D.; Collin, D.; Czernichow, P.; Lévy-Marchal, C. Dynamic change in adiposity from fetal to postnatal life is involved in the metabolic syndrome associated with reduced fetal growth. Diabetologia 2005, 48, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Woo Baidal, J.A.; Locks, L.M.; Cheng, E.R.; Blake-Lamb, T.L.; Perkins, M.E.; Taveras, E.M. Risk Factors for Childhood Obesity in the First 1,000 Days: A Systematic Review. Am. J. Prev. Med. 2016, 50, 761–779. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Lin, H.Y.; Wang, C.H.; Su, B.H.; Lin, C.C. Association of preterm birth and small for gestational age with metabolic outcomes in children and adolescents: A population-based cohort study from Taiwan. Pediatr. Neonatol. 2018, 59, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Bozzetti, V.; Tagliabue, P.E. Enteral feeding of intrauterine growth restriction preterm infants: Theoretical risks and practical implications. Pediatr. Med. Chir. 2017, 39, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudehope, D.; Vento, M.; Bhutta, Z.; Pachi, P. Nutritional requirements and feeding recommendations for small for gestational age infants. J. Pediatr. 2013, 162, S81–S89. [Google Scholar] [CrossRef]
- Milovanovic, I.; Njuieyon, F.; Deghmoun, S.; Chevenne, D.; Levy-Marchal, C.; Beltrand, J. Innate small babies are metabolically healthy children. J. Clin. Endocrinol. Metab. 2012, 97, 4407–4413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Recio Linares, A.; Bezanilla López, C.; Barasoain Millán, A.; Domínguez Uribe-Echevarría, M.; García Rodríguez, C.; Torrejón López, M.; Pérez Fernández, E.; Botija Arcos, G.; Barrio Merino, A. Longitudinal study of the newborn small for gestational age. Growth recovery and conditioning factors. Nutr. Hosp. 2022, 39, 520–529. [Google Scholar] [CrossRef]
- Campisi, S.C.; Carbone, S.E.; Zlotkin, S. Catch-Up Growth in Full-Term Small for Gestational Age Infants: A Systematic Review. Adv. Nutr. 2019, 10, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Castanys-Muñoz, E.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.; van der Beek, E.M.; et al. Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr. 2017, 106, 1230–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singhal, A.; Cole, T.J.; Fewtrell, M.; Kennedy, K.; Stephenson, T.; Elias-Jones, A.; Lucas, A. Promotion of faster weight gain in infants born small for gestational age: Is there an adverse effect on later blood pressure? Circulation 2007, 115, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Kennedy, K.; Lanigan, J.; Fewtrell, M.; Cole, T.J.; Stephenson, T.; Elias-Jones, A.; Weaver, L.T.; Ibhanesebhor, S.; MacDonald, P.D.; et al. Nutrition in infancy and long-term risk of obesity: Evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 2010, 92, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, A.C.T.; Cunha, L.; Vieira, N.S.A.; Oliveira Moreira, L.M.; Oliveira, P.R.; Lyra, P.P.R.; Alves, C.A.D. Breastfeeding in children born small for gestational age and future nutritional and metabolic outcomes: A systematic review. J. Pediatr. 2019, 95, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Soto, N.; Bazaes, R.A.; Peña, V.; Salazar, T.; Avila, A.; Iñiguez, G.; Ong, K.K.; Dunger, D.B.; Mericq, M.V. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: Results from a prospective cohort. J. Clin. Endocrinol. Metab. 2003, 88, 3645–3650. [Google Scholar] [CrossRef]
- Leunissen, R.W.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009, 301, 2234–2242. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Gamble, G.D.; Crowther, C.A.; Bloomfield, F.H.; Agosti, M.; Atkinson, S.A.; Biasini, A.; Embleton, N.D.; Fewtrell, M.S.; Lamy-Filho, F.; et al. Sex-Specific Effects of Nutritional Supplements for Infants Born Early or Small: An Individual Participant Data Meta-Analysis (ESSENCE IPD-MA) I-Cognitive Function and Metabolic Risk. Nutrients 2022, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Groene, S.G.; Stegmeijer, K.J.J.; Tan, R.; Steggerda, S.J.; Haak, M.C.; Slaghekke, F.; Roest, A.A.W.; Heijmans, B.T.; Lopriore, E.; van Klink, J.M.M. Long-term effects of selective fetal growth restriction (LEMON): A cohort study of neurodevelopmental outcome in growth discordant identical twins in the Netherlands. Lancet Child Adolesc. Health 2022, 6, 624–632. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Guidelines. In Twin and Triplet Pregnancy; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Good clinical practice advice: Management of twin pregnancy. Int. J. Gynaecol. Obstet. 2019, 144, 330–337. [CrossRef] [PubMed] [Green Version]
- Khalil, A.; Beune, I.; Hecher, K.; Wynia, K.; Ganzevoort, W.; Reed, K.; Lewi, L.; Oepkes, D.; Gratacos, E.; Thilaganathan, B.; et al. Consensus definition and essential reporting parameters of selective fetal growth restriction in twin pregnancy: A Delphi procedure. Ultrasound Obstet. Gynecol. 2019, 53, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, N.S.; Rebarber, A.; Klauser, C.K.; Roman, A.S.; Saltzman, D.H. Intrauterine growth restriction in twin pregnancies: Incidence and associated risk factors. Am. J. Perinatol. 2011, 28, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, T.E.; Wilkins, R.; Sherman, G.J. Birth weight percentiles by gestational age in Canada. Obstet. Gynecol. 1993, 81, 39–48. [Google Scholar]
- Secher, N.J.; Kaern, J.; Hansen, P.K. Intrauterine growth in twin pregnancies: Prediction of fetal growth retardation. Obstet. Gynecol. 1985, 66, 63–68. [Google Scholar]
- Sukhwani, M.; Antolín, E.; Herrero, B.; Rodríguez, R.; de la Calle, M.; López, F.; Bartha, J.L. Management and perinatal outcome of selective intrauterine growth restriction in monochorionic pregnancies. J. Matern. Fetal Neonatal Med. 2021, 34, 3838–3843. [Google Scholar] [CrossRef]
- Lapillonne, A.; Griffin, I.J. Feeding preterm infants today for later metabolic and cardiovascular outcomes. J. Pediatr. 2013, 162, S7–S16. [Google Scholar] [CrossRef]
- Embleton, N.D.; Moltu, S.J.; Lapillonne, A.; van den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper From the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2022, 76, 248–268. [Google Scholar] [CrossRef]
- Kosmeri, C.; Giapros, V.; Gounaris, A.; Sokou, R.; Siomou, E.; Rallis, D.; Makis, A.; Baltogianni, M. Are the current feeding volumes adequate for the growth of very preterm neonates? Br. J. Nutr. 2023, 1–5. [Google Scholar] [CrossRef]
- Gounaris, A.K.; Sokou, R.; Gounari, E.; Panagiotounakou, P.; Grivea, I.N. Post-natal growth of very preterm neonates. Lancet Child Adolesc. Health 2022, 6, e9–e10. [Google Scholar] [CrossRef]
- Gounaris, A.; Sokou, R.; Theodoraki, M.; Gounari, E.; Panagiotounakou, P.; Antonogeorgos, G.; Ioakeimidis, G.; Parastatidou, S.; Konstantinidi, A.; Grivea, I.N. “Aggressive” Feeding of Very Preterm Neonates and Body Mass Index at School Age. Nutrients 2021, 13, 1901. [Google Scholar] [CrossRef]
- Embleton, N.E.; Pang, N.; Cooke, R.J. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics 2001, 107, 270–273. [Google Scholar] [CrossRef]
- Dutta, S.; Singh, B.; Chessell, L.; Wilson, J.; Janes, M.; McDonald, K.; Shahid, S.; Gardner, V.A.; Hjartarson, A.; Purcha, M.; et al. Guidelines for feeding very low birth weight infants. Nutrients 2015, 7, 423–442. [Google Scholar] [CrossRef] [Green Version]
- Karagianni, P.; Briana, D.D.; Mitsiakos, G.; Elias, A.; Theodoridis, T.; Chatziioannidis, E.; Kyriakidou, M.; Nikolaidis, N. Early versus delayed minimal enteral feeding and risk for necrotizing enterocolitis in preterm growth-restricted infants with abnormal antenatal Doppler results. Am. J. Perinatol. 2010, 27, 367–373. [Google Scholar] [CrossRef]
- Leaf, A.; Dorling, J.; Kempley, S.; McCormick, K.; Mannix, P.; Linsell, L.; Juszczak, E.; Brocklehurst, P. Early or delayed enteral feeding for preterm growth-restricted infants: A randomized trial. Pediatrics 2012, 129, e1260–e1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Elburg, R.M.; van den Berg, A.; Bunkers, C.M.; van Lingen, R.A.; Smink, E.W.; van Eyck, J.; Fetter, W.P. Minimal enteral feeding, fetal blood flow pulsatility, and postnatal intestinal permeability in preterm infants with intrauterine growth retardation. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F293–F296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmaaboud, M.; Mohammed, A. Early Versus Late Minimal Enteral Feeding in Weeks Preterm Growth-Restricted neonates with Abnormal Antenatal Doppler Studies. J. Matern. Fetal Neonatal Med. 2012, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Young, L.; Oddie, S.J.; McGuire, W. Delayed introduction of progressive enteral feeds to prevent necrotising enterocolitis in very low birth weight infants. Cochrane Database Syst. Rev. 2022, 1, Cd001970. [Google Scholar] [CrossRef] [PubMed]
- Kempley, S.; Gupta, N.; Linsell, L.; Dorling, J.; McCormick, K.; Mannix, P.; Juszczak, E.; Brocklehurst, P.; Leaf, A. Feeding infants below 29 weeks’ gestation with abnormal antenatal Doppler: Analysis from a randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F6–F11. [Google Scholar] [CrossRef] [PubMed]
- Fallon, E.M.; Nehra, D.; Potemkin, A.K.; Gura, K.M.; Simpser, E.; Compher, C.; Puder, M.A.S.P.E.N. clinical guidelines: Nutrition support of neonatal patients at risk for necrotizing enterocolitis. JPEN J. Parenter. Enter. Nutr. 2012, 36, 506–523. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.; Embleton, N.D.; McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 2019, 7, Cd002971. [Google Scholar] [CrossRef] [Green Version]
- Hair, A.B.; Hawthorne, K.M.; Chetta, K.E.; Abrams, S.A. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res. Notes 2013, 6, 459. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.H.; Thomas, P.; Peabody, J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003, 111, 986–990. [Google Scholar] [CrossRef]
- Visuthranukul, C.; Abrams, S.A.; Hawthorne, K.M.; Hagan, J.L.; Hair, A.B. Premature small for gestational age infants fed an exclusive human milk-based diet achieve catch-up growth without metabolic consequences at 2 years of age. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F242–F247. [Google Scholar] [CrossRef] [Green Version]
- Senterre, T.; Rigo, J. Optimizing early nutritional support based on recent recommendations in VLBW infants and postnatal growth restriction. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 536–542. [Google Scholar] [CrossRef]

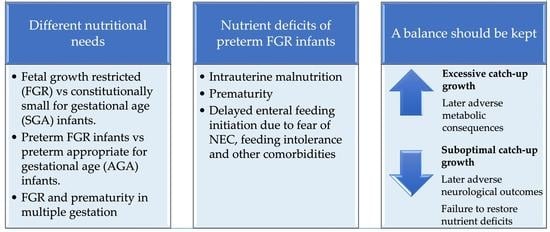
| Considerations in Nutritional Management of Preterm FGR Infants |
|---|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmeri, C.; Giapros, V.; Rallis, D.; Balomenou, F.; Serbis, A.; Baltogianni, M. Classification and Special Nutritional Needs of SGA Infants and Neonates of Multiple Pregnancies. Nutrients 2023, 15, 2736. https://doi.org/10.3390/nu15122736
Kosmeri C, Giapros V, Rallis D, Balomenou F, Serbis A, Baltogianni M. Classification and Special Nutritional Needs of SGA Infants and Neonates of Multiple Pregnancies. Nutrients. 2023; 15(12):2736. https://doi.org/10.3390/nu15122736
Chicago/Turabian StyleKosmeri, Chrysoula, Vasileios Giapros, Dimitrios Rallis, Foteini Balomenou, Anastasios Serbis, and Maria Baltogianni. 2023. "Classification and Special Nutritional Needs of SGA Infants and Neonates of Multiple Pregnancies" Nutrients 15, no. 12: 2736. https://doi.org/10.3390/nu15122736