Astragalus membranaceus Enhances Myotube Hypertrophy through PI3K-Mediated Akt/mTOR Signaling Phosphorylation
Abstract
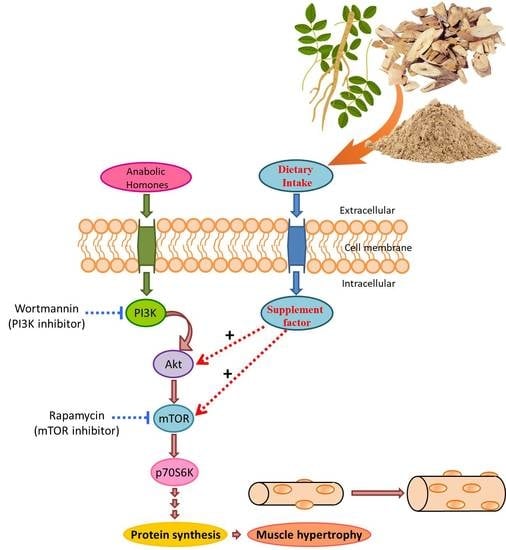
:1. Introduction
2. Materials and Methods
2.1. Skeletal Muscle Cell Culture
2.2. Astragalus membranaceus (AM) and Chemical Reagents
2.3. Cytotoxicity of Astragalus membranaceus (AM)
2.4. Myotube Diameter
2.5. Western Blotting
2.6. Astragalus Analysis of Astragalus membranaceus
2.7. Statistical Analyses
3. Results
3.1. Cytotoxicity of Astragalus membranaceus on Myotubes
3.2. Promotion of Myotube Hypertrophy by Astragalus membranaceus
3.3. Astragalus membranaceus and the PI3K/Akt/mTOR Pathway
3.4. AM-Induced Phosphorylation of Akt on Ser473
3.5. AM-Induced Phosphorylation of mTOR on Ser2448
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging. Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Dionyssiotis, Y. Sarcopenia in the Elderly. Eur. Endocrinol. 2019, 15, 13–14. [Google Scholar] [CrossRef]
- Evans, M.; Guthrie, N.; Pezzullo, J.; Sanli, T.; Fielding, R.A.; Bellamine, A. Efficacy of a novel formulation of L-carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: A randomized, double-blind placebo-controlled study. Nutr. Metab. 2017, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, P.L.; Morales, J.S.; Emanuele, E.; Pareja-Galeano, H.; Lucia, A. Supplements with purported effects on muscle mass and strength. Eur. J. Nutr. 2019, 58, 2983–3008. [Google Scholar] [CrossRef]
- Go, G.Y.; Jo, A.; Seo, D.W.; Kim, W.Y.; Kim, Y.K.; So, E.Y.; Chen, Q.; Kang, J.S.; Bae, G.U.; Lee, S.J. Ginsenoside Rb1 and Rb2 upregulate Akt/mTOR signaling-mediated muscular hypertrophy and myoblast differentiation. J. Ginseng. Res. 2020, 44, 435–441. [Google Scholar] [CrossRef]
- Lin, Y.A.; Li, Y.R.; Chang, Y.C.; Hsu, M.C.; Chen, S.T. Activation of IGF-1 pathway and suppression of atrophy related genes are involved in Epimedium extract (icariin) promoted C2C12 myotube hypertrophy. Sci. Rep. 2021, 11, 10790. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Seo, E.; Yeon, M.; Kim, M.S.; Hur, H.J.; Oh, B.C.; Jun, H.S. Anti-aging effects of Schisandrae chinensis fructus extract: Improvement of insulin sensitivity and muscle function in aged mice. Evid.-Based. Complement. Alternat. Med. 2019, 2019, 5642149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalé, A.; Cloutier, G.J.; Hau, C.; Phillips, E.M.; Dallal, G.E.; Fielding, R.A. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 682–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell. Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Timmer, L.T.; Hoogaars, W.M.H.; Jaspers, R.T. The role of IGF-1 signaling in skeletal muscle atrophy. Adv. Exp. Med. Biol. 2018, 1088, 109–137. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, F.Y. Shen Nong Ben Cao Jing, Huangqi; Shanxi Science and Technology Press: Taiyuan, China, 2017; pp. 112–113. ISBN 9787537755153. [Google Scholar]
- Yin, M.; Yang, M.; Chu, S.; Li, R.; Zhao, Y.; Peng, H.; Zhan, Z.; Sun, H.F. Quality analysis of different specification grades of Astragalus membranaceus var. mongholicus (Huangqi) from Hunyuan, Shanxi. J. AOAC Int. 2019, 102, 734–740. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.Y.; Fu, Y.J.; Ma, W.; Peng, X.; Tan, S.N.; Efferth, T. Efficient production of isoflavonoids by Astragalus membranaceus hairy root cultures and evaluation of antioxidant activities of extracts. J. Agric. Food. Chem. 2014, 62, 12649–12658. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, H.; Mu, Y.; Sun, M.; Liu, P. Pharmacological effects of Astragaloside IV: A literature review. J. Tradit. Chin. Med. 2013, 33, 413–416. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Committee. Chemical Drugs and Biological Agents. In Pharmacopoeia of the People’s Republic of China, 2010 ed.; China Medical Science and Technology; Chinese Medical Science Press: Beijing, China, 2010; pp. 124–125. ISBN 9787506744393. [Google Scholar]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, H.; Wang, H. Astragaloside IV alleviates myocardial damage induced by type 2 diabetes via improving energy metabolism. Mol. Med. Rep. 2019, 20, 4612–4622. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yang, F.; Liu, J.; Zhang, M.; Sun, J.; Xiao, Y.; Xiao, Z.; Niu, H.; Ma, R.; Wang, Y.; et al. Astragaloside IV reduces cardiomyocyte apoptosis in a murine model of coxsackievirus B3-induced viral myocarditis. Exp. Anim. 2019, 68, 549–558. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Zuo, D.; Li, Z.; Liu, H.; Dai, Z.; Cai, J.; Pang, L.; Wu, Y. Astragaloside IV inhibits doxorubicin-induced cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway via activating the PI3K/Akt pathway. Chem. Pharm. Bull. 2014, 62, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Xie, Y.; Shen, E.; Li, G.G.; Yu, Y.; Zhang, C.B.; Yang, Y.; Zou, Y.; Ge, J.; Chen, R.; et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur. J. Pharmacol. 2011, 658, 168–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Li, S.; Li, Z.H.; Lam, C.O.; Hong, S.J.; Kwan, Y.W.; Chan, S.W.; Leung, G.P.; Lee, S.M. Pro-angiogenic activity of astragaloside IV in HUVECs in vitro and zebrafish in vivo. Mol. Med. Rep. 2012, 5, 805–811. [Google Scholar] [CrossRef]
- Wang, S.G.; Xu, Y.; Chen, J.D.; Yang, C.H.; Chen, X.H. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 2013, 18, 12809–12819. [Google Scholar] [CrossRef] [Green Version]
- Ji, K.; Chen, J.; Hu, J.; Xue, Y.; Yin, R.; Lu, Q.; Wu, W.; Wang, G.; Wang, X.; Song, X.; et al. The protective effect of astragaloside IV against benzo[a]pyrene induced endothelial progenitor cell dysfunction. Life Sci. 2015, 132, 13–19. [Google Scholar] [CrossRef]
- Liu, X.; Min, W. Protective effects of astragaloside against ultraviolet A-induced photoaging in human fibroblasts. Zhong Xi Yi Jie He Xue Bao 2011, 9, 328–332. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Huang, H.; Gao, W.; Zhuang, C.; Li, B.; Zhou, P.; Kong, D. Anti-hepatitis B virus activities of astragaloside IV isolated from Radix Astragali. Biol. Pharm. Bull. 2009, 32, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.; Wu, S.Y.; Wang, G.F.; Zhang, J.J.; Pang, J.X.; Liu, Z.Q.; Xu, W.; Wu, S.G.; Rao, J.J. Effect of astragaloside IV on hepatic glucose-regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Phytother. Res. 2010, 24, 219–224. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wang, Z.; Yu, S.; Long, T.; Zhou, X.; Bao, Y. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci. Rep. 2017, 7, 44822. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Jing, C.; Shi, W. Effects of Chinese herbal recipes on immunity in immunosuppressive mice. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Luo, S.H.; Huang, D.C.; Cheng, S.J.; Cao, C.J.; Chen, G.T. Immunomodulatory activities of proteins from Astragalus membranaceus waste. J. Sci. Food Agric. 2019, 99, 4174–4181. [Google Scholar] [CrossRef]
- Yeh, T.S.; Chuang, H.L.; Huang, W.C.; Chen, Y.M.; Huang, C.C.; Hsu, M.C. Astragalus membranaceus improves exercise performance and ameliorates exercise-induced fatigue in trained mice. Molecules 2014, 19, 2793–2807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Wu, K.; Mao, X.; Wu, Y.; Ouyang, J. Astragalus polysaccharide improves insulin sensitivity in KKAy mice: Regulation of PKB/GLUT4 signaling in skeletal muscle. J. Ethnopharmacol. 2010, 127, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.R.; Cook, S.A.; Buranasombati, C.; Rosenberg, M.A.; Rosenzweig, A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am. J. Physiol. Cell Physiol. 2009, 297, C1124–C1132. [Google Scholar] [CrossRef] [Green Version]
- Aswad, H.; Jalabert, A.; Rome, S. Depleting extracellular vesicles from fetal bovine serum alters proliferation and differentiation of skeletal muscle cells in vitro. BMC Biotechnol. 2016, 16, 32. [Google Scholar] [CrossRef] [Green Version]
- Sasai, N.; Agata, N.; Inoue-Miyazu, M.; Kawakami, K.; Kobayashi, K.; Sokabe, M.; Hayakawa, K. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve 2010, 41, 100–106. [Google Scholar] [CrossRef]
- Marabita, M.; Baraldo, M.; Solagna, F.; Ceelen, J.J.M.; Sartori, R.; Nolte, H.; Nemazanyy, I.; Pyronnet, S.; Kruger, M.; Pende, M. S6K1 is required for increasing skeletal muscle force during hypertrophy. Cell Rep. 2016, 17, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Peters, E.L.; van der Linde, S.M.; Vogel, I.S.P.; Haroon, M.; Offringa, C.; de Wit, G.M.J.; Koolwijk, P.; van der Laarse, W.J.; Jaspers, R.T. IGF-1 attenuates hypoxia-induced atrophy but inhibits myoglobin expression in C2C12 skeletal muscle myotubes. Int. J. Mol. Sci. 2017, 18, 1889. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.Y.; Tai, C.C.; Li, Y.H.; Wu, J.L. Progranulin compensates for blocked IGF-1 signaling to promote myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS Lett. 2012, 586, 3485–3492. [Google Scholar] [CrossRef] [Green Version]
- Dennis, P.B.; Jaeschke, A.; Saitoh, M.; Fowler, B.; Kozma, S.C.; Thomas, G. Mammalian TOR: A homeostatic ATP sensor. Science 2001, 294, 1102–1105. [Google Scholar] [CrossRef]
- Chiang, G.G.; Abraham, R.T. Phosphorylation of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by p70S6 kinase. J. Biol. Chem. 2005, 280, 25485–25490. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, V.C.; Markworth, J.F.; Cameron-Smith, D. Considerations on mTOR regulation at serine 2448: Implications for muscle metabolism studies. Cell Mol. Life Sci. 2017, 74, 2537–2545. [Google Scholar] [CrossRef]
- Yu, T.; Wang, M.; Wen, Y.; Cao, Y.; Shen, G.; Jiang, X.; Wu, J.; Lu, W.; Jin, X. Activation of mammalian target of rapamycin induces lipid accumulation in the diaphragm of ventilated rats and hypoxia-treated C2C12 cells. J. Surg. Res. 2018, 225, 82–89. [Google Scholar] [CrossRef]
- Yeh, T.S.; Hsu, C.C.; Yang, S.C.; Hsu, M.C.; Liu, J.F. Angelica sinensis promotes myotube hypertrophy through the PI3K/Akt/mTOR pathway. BMC Complement. Altern. Med. 2014, 14, 144. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, X.; Peng, X.; Sun, L.; Jia, S.; Wang, P.; Ma, S.; Zhao, H. Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes. Exp. Ther. Med. 2017, 14, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Basualto-Alarcón, C.; Jorquera, G.; Altamirano, F.; Jaimovich, E.; Estrada, M. Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med. Sci. Sports. Exerc. 2013, 45, 1712–1720. [Google Scholar] [CrossRef]
- Blomstrand, E.; Eliasson, J.; Karlsson, H.K.; Kohnke, R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J. Nutr. 2006, 136, 269S–273S. [Google Scholar] [CrossRef]
- Farnfield, M.M.; Breen, L.; Carey, K.A.; Garnham, A.; Cameron-Smith, D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl. Physiol. Nutr. Metab. 2012, 37, 21–30. [Google Scholar] [CrossRef]
- Lu, L.; Wang, D.T.; Shi, Y.; Yin, Y.; Wei, L.B.; Zou, Y.C.; Huang, B.; Zhao, Y.; Wang, M.; Wan, H.; et al. Astragalus polysaccharide improves muscle atrophy from dexamethasone- and peroxide-induced injury in vitro. Int. J. Biol. Macromol. 2013, 61, 7–16. [Google Scholar] [CrossRef]
- Lee, S.Y.; Go, G.Y.; Vuong, T.A.; Kim, J.W.; Lee, S.; Jo, A.; An, J.M.; Kim, S.N.; Seo, D.W.; Kim, J.S.; et al. Black ginseng activates Akt signaling, thereby enhancing myoblast differentiation and myotube growth. J. Ginseng. Res. 2018, 42, 116–121. [Google Scholar] [CrossRef]
- Edström, E.; Altun, M.; Hägglund, M.; Ulfhake, B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 663–674. [Google Scholar] [CrossRef]
- Kim, D.H.; Bang, E.; Ha, S.; Jung, H.J.; Choi, Y.J.; Yu, B.P.; Chung, H.Y. Organ-differential Roles of Akt/FoxOs Axis as a Key Metabolic Modulator during Aging. Aging. Dis. 2021, 12, 1713–1728. [Google Scholar] [CrossRef]



| Identification | Astragalus membranaceus Sample (mg/g) |
|---|---|
| Astragaloside I | 0.212 |
| Astragaloside II | 0.431 |
| Astragaloside III | 0.076 |
| Astragaloside IV | 0.507 |
| Total Astragalosides | 1.226 |
| Concentration of Treated Astragalus membranaceus (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Con | 1 | 10 | 102 | 103 | 104 | 105 | 106 | |
| 24 h | 100.00 ± 4.69 | 98.44 ± 5.41 | 97.25 ± 4.45 | 97.23 ± 4.16 | 96.36 ± 5.03 | 93.18 ± 3.02 | 89.56 ± 1.33 | 87.94 ± 4.11 |
| 48 h | 100.00 ± 4.74 | 100.59 ± 4.88 | 101.19 ± 4.23 | 100.08 ± 3.78 | 97.38 ± 3.31 | 92.99 ± 3.01 | 88.15 ± 2.24 * | 81.91 ± 4.19 * |
| 72 h | 100.00 ± 3.75 | 99.25 ± 3.49 | 101.31 ± 5.21 | 99.34 ± 4.86 | 99.10 ± 3.92 | 92.56 ± 3.59 | 88.11 ± 5.26 * | 82.64 ± 4.53 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, T.-S.; Lei, T.-H.; Liu, J.-F.; Hsu, M.-C. Astragalus membranaceus Enhances Myotube Hypertrophy through PI3K-Mediated Akt/mTOR Signaling Phosphorylation. Nutrients 2022, 14, 1670. https://doi.org/10.3390/nu14081670
Yeh T-S, Lei T-H, Liu J-F, Hsu M-C. Astragalus membranaceus Enhances Myotube Hypertrophy through PI3K-Mediated Akt/mTOR Signaling Phosphorylation. Nutrients. 2022; 14(8):1670. https://doi.org/10.3390/nu14081670
Chicago/Turabian StyleYeh, Tzu-Shao, Tze-Huan Lei, Jen-Fang Liu, and Mei-Chich Hsu. 2022. "Astragalus membranaceus Enhances Myotube Hypertrophy through PI3K-Mediated Akt/mTOR Signaling Phosphorylation" Nutrients 14, no. 8: 1670. https://doi.org/10.3390/nu14081670