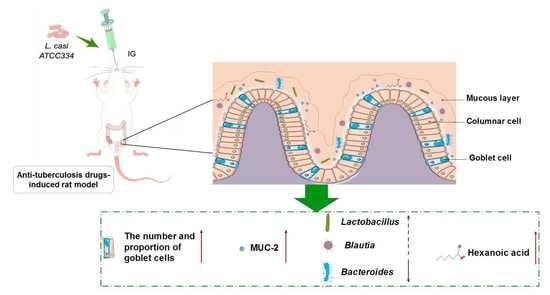
Lactobacillus casei Improve Anti-Tuberculosis Drugs-Induced Intestinal Adverse Reactions in Rat by Modulating Gut Microbiota and Short-Chain Fatty Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Reagent Preparation
2.3. Grouping and Modeling
2.4. Sample Collection
2.5. Intestinal Histopathology
2.6. Intestinal Immune Function and Pro-Inflammatory Factors
2.7. DNA Extraction, 16S rRNA Gene Amplification, and Sequencing
2.8. Short-Chain Fatty Acids Measurements of Colon Contents
2.9. Statistical Analysis
3. Results
3.1. Intestinal Histopathological Analysis
3.2. Analysis of Related Factors of Intestinal Immunity and Inflammation
3.3. Analysis on Intestinal Microflora of Anti-TB Drugs and Lactobacillus casei
3.3.1. Alpha Diversity and Beta Diversity Analysis
3.3.2. The Effect of L. casei ATCC334 on Microbiome Changes Induced by Anti-TB Drugs
3.3.3. The Effect of Lactobacillus casei on SCFAs Changes Induced by Anti-TB Drugs
3.3.4. The Effect of Anti-TB Drugs and Lactobacillus casei on Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suárez, I.; Fünger, S.M.; Kröger, S.; Rademacher, J.; Fätkenheuer, G.; Rybniker, J. The Diagnosis and Treatment of Tuberculosis. Dtsch. Arztebl. Int. 2019, 116, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Tang, S.; Xia, Y.; Wang, X.; Yuan, Y.; Hu, D.; Liu, F.; Wu, S.; Zhang, Y.; Yang, Z.; et al. Adverse reactions due to directly observed treatment strategy therapy in Chinese tuberculosis patients: A prospective study. PLoS ONE 2013, 8, e65037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farazi, A.; Sofian, M.; Jabbariasl, M. Adverse reactions to antituberculosis drugs in Iranian tuberculosis patients. Tuberc. Res. Treat. 2014, 2014, 412893. [Google Scholar] [CrossRef] [PubMed]
- El Hamdouni, M.; Ahid, S.; Bourkadi, J.E.; Benamor, J.; Hassar, M.; Cherrah, Y. Incidence of adverse reactions caused by first-line anti-tuberculosis drugs and treatment outcome of pulmonary tuberculosis patients in Morocco. Infection 2020, 48, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, G.S.; Guaraldo, L.; Engstrom, E.M.; Theme Filha, M.M.; Souza-Santos, R.; Vasconcelos, A.G.; Rozenfeld, S. Adverse reactions to antituberculosis drugs in Manguinhos, Rio de Janeiro, Brazil. Clinics 2013, 68, 329–337. [Google Scholar] [CrossRef]
- Dethlefsen, L.; Huse, S.; Sogin, M.L.; Relman, D.A. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008, 6, e280. [Google Scholar] [CrossRef]
- Silverman, M.A.; Konnikova, L.; Gerber, J.S. Impact of Antibiotics on Necrotizing Enterocolitis and Antibiotic-Associated Diarrhea. Gastroenterol. Clin. N. Am. 2017, 46, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, X.; Zhang, Y.; Zheng, K.; Xiang, Q.; Chen, N.; Chen, Z.; Zhang, N.; Zhu, J.; He, Q. Antibiotic-Induced Disruption of Gut Microbiota Alters Local Metabolomes and Immune Responses. Front. Cell. Infect. Microbiol. 2019, 9, 99. [Google Scholar] [CrossRef]
- Binder, H.J. Role of colonic short-chain fatty acid transport in diarrhea. Annu. Rev. Physiol. 2010, 72, 297–313. [Google Scholar] [CrossRef]
- Namasivayam, S.; Maiga, M.; Yuan, W.; Thovarai, V.; Costa, D.L.; Mittereder, L.R.; Wipperman, M.F.; Glickman, M.S.; Dzutsev, A.; Trinchieri, G.; et al. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome 2017, 5, 71. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.H.; Chiu, C.H.; Kong, M.S.; Chang, C.J.; Chen, C.C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Zhao, C.; Du, Y.; Zhang, Y.; Zhao, M.; Zhao, Q. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: Systematic review with network meta-analysis. United Eur. Gastroenterol. J. 2018, 6, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Pinart-Gilberga, M.; Jones, M.; Hoyles, L.; McCartney, A.L.; Gibson, G.R. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J. Appl. Microbiol. 2007, 102, 1026–1032. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Kawai, M.; Kikuchi-Hayakawa, H.; Suda, K.; Ishikawa, H.; Gondo, Y.; Shimizu, K.; Matsuki, T.; et al. Fermented Milk Containing Lactobacillus casei Strain Shirota Preserves the Diversity of the Gut Microbiota and Relieves Abdominal Dysfunction in Healthy Medical Students Exposed to Academic Stress. Appl. Environ. Microbiol. 2016, 82, 3649–3658. [Google Scholar] [CrossRef] [Green Version]
- Rieu, A.; Aoudia, N.; Jego, G.; Chluba, J.; Yousfi, N.; Briandet, R.; Deschamps, J.; Gasquet, B.; Monedero, V.; Garrido, C.; et al. The biofilm mode of life boosts the anti-inflammatory properties of Lactobacillus. Cell. Microbiol. 2014, 16, 1836–1853. [Google Scholar] [CrossRef]
- Ren, M.; Li, H. Centenarian-Sourced Lactobacillus casei Combined with Dietary Fiber Complex Ameliorates Brain and Gut Function in Aged Mice. Nutrients 2022, 14, 324. [Google Scholar] [CrossRef]
- Yao, G.; Cao, C.; Zhang, M.; Kwok, L.Y.; Zhang, H.; Zhang, W. Lactobacillus casei Zhang exerts probiotic effects to antibiotic-treated rats. Comput. Struct. Biotechnol. J. 2021, 19, 5888–5897. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, S.; Liu, J.; Zhang, J.; Zhang, C.; Hao, H.; Sun, Y.; Cai, J.; Yang, Y.; Ma, Y.; et al. Efficacy of proprietary Lactobacillus casei for anti-tuberculosis associated gastrointestinal adverse reactions in adult patients: A randomized, open-label, dose-response trial. Food Funct. 2020, 11, 370–377. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef]
- Wang, C.; Fan, R.Q.; Zhang, Y.X.; Nie, H.; Li, K. Naringenin protects against isoniazid- and rifampicin-induced apoptosis in hepatic injury. World J. Gastroenterol. 2016, 22, 9775–9783. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.; Keeney, K.M.; Menendez, A.; Bergstrom, K.S.; Gill, N.; Russell, S.L.; Vallance, B.A.; Finlay, B.B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, J.; Bobe, A.M.; Miyoshi, S.; Huang, Y.; Hubert, N.; Delmont, T.O.; Eren, A.M.; Leone, V.; Chang, E.B. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep. 2017, 20, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Örtqvist, A.K.; Lundholm, C.; Halfvarson, J.; Ludvigsson, J.F. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: A population-based study. Gut 2019, 68, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Holzheimer, R.G. Antibiotic induced endotoxin release and clinical sepsis: A review. J. Chemother. 2001, 13, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Q.; Liu, B.; Dong, J.; Sun, L.; Zhu, Y.; Su, H.; Yang, J.; Yang, F.; Chen, X.; et al. Gut microbiota associated with pulmonary tuberculosis and dysbiosis caused by anti-tuberculosis drugs. J. Infect. 2019, 78, 317–322. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Ladinsky, M.S. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuddenham, S.; Sears, C.L. The intestinal microbiome and health. Curr. Opin. Infect. Dis. 2015, 28, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Liu, Y.; Wu, P.; Luo, D.X.; Sun, Q.; Zheng, H.; Hu, R.; Pandol, S.J.; Li, Q.F.; Han, Y.P.; et al. Alternation of Gut Microbiota in Patients with Pulmonary Tuberculosis. Front. Physiol. 2017, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Lankelma, J.M.; Hugenholtz, F.; Belzer, C.; de Vos, W.M.; Wiersinga, W.J. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 2019, 74, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Wipperman, M.F.; Fitzgerald, D.W.; Juste, M.A.J.; Taur, Y.; Namasivayam, S.; Sher, A.; Bean, J.M.; Bucci, V.; Glickman, M.S. Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci. Rep. 2017, 7, 10767. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1792–1801. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Richard, M.L.; Liguori, G.; Lamas, B.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Pierluigi Di Simone, M.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes 2018, 9, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G. Lactobacillus plantarum KLDS1.0344 and Lactobacillus acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.Z.; Zhang, R.N.; He, C.X.; Chen, G.Y.; Liu, C.; Chen, Y.W.; Fan, J.G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Keenan, M.J.; Marco, M.L.; Ingram, D.K.; Martin, R.J. Improving healthspan via changes in gut microbiota and fermentation. AGE 2015, 37, 98. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, L.; Hou, M.; Gao, T.; Sun, J.; Luo, H.; Wang, F.; Zhong, F.; Ma, A.; Cai, J. Lactobacillus casei Improve Anti-Tuberculosis Drugs-Induced Intestinal Adverse Reactions in Rat by Modulating Gut Microbiota and Short-Chain Fatty Acids. Nutrients 2022, 14, 1668. https://doi.org/10.3390/nu14081668
Li Y, Zhao L, Hou M, Gao T, Sun J, Luo H, Wang F, Zhong F, Ma A, Cai J. Lactobacillus casei Improve Anti-Tuberculosis Drugs-Induced Intestinal Adverse Reactions in Rat by Modulating Gut Microbiota and Short-Chain Fatty Acids. Nutrients. 2022; 14(8):1668. https://doi.org/10.3390/nu14081668
Chicago/Turabian StyleLi, Yue, Liangjie Zhao, Meiling Hou, Tianlin Gao, Jin Sun, Hao Luo, Fengdan Wang, Feng Zhong, Aiguo Ma, and Jing Cai. 2022. "Lactobacillus casei Improve Anti-Tuberculosis Drugs-Induced Intestinal Adverse Reactions in Rat by Modulating Gut Microbiota and Short-Chain Fatty Acids" Nutrients 14, no. 8: 1668. https://doi.org/10.3390/nu14081668