Effect of an Acute Insect Preload vs. an Almond Preload on Energy Intake, Subjective Food Consumption and Intestinal Health in Healthy Young Adults
Abstract
:1. Introduction
2. Materials and Methods
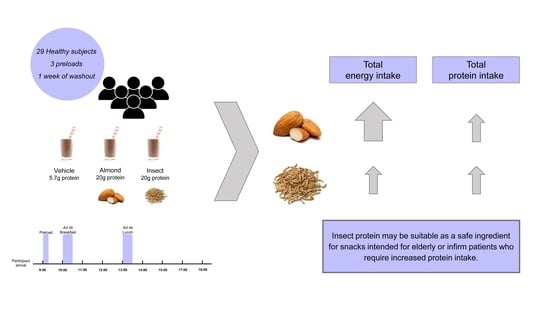
2.1. Subjects
2.2. Experimental Design
2.3. Preloads
2.4. Subjective Ratings of Appetite
2.5. Statistical Analysis
3. Results
3.1. Energy and Protein Intakes
3.2. Subjective Ratings of Appetite
3.3. Preload Palatability and Correlation with Food Intake
3.4. Digestive Health and Its Correlation with Food Intake
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, G.H.; Moore, S.E. Dietary Proteins in the Regulation of Food Intake and Body Weight in Humans. J. Nutr. 2004, 134, 974–979. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. The significance of protein in food intake and body weight regulation. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 635–638. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Ellis, V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br. J. Nutr. 2010, 104, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.H.; Tecimer, S.N.; Shah, D.; Zafar, T.A. Protein Source, Quantity, and Time of Consumption Determine the Effect of Proteins on Short-Term Food Intake in Young Men. J. Nutr. 2004, 134, 3011–3015. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.L.; Millward, D.J.; Long, S.J.; Morgan, L.M. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br. J. Nutr. 2003, 89, 239–248. [Google Scholar] [CrossRef]
- Abou-Samra, R.; Keersmaekers, L.; Brienza, D.; Mukherjee, R.; Macé, K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr. J. 2011, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad Libitum energy intake. J. Clin. Endocrinol. Metab. 2006, 91, 2913–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowen, J.; Noakes, M.; Trenerry, C.; Clifton, P.M. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J. Clin. Endocrinol. Metab. 2006, 91, 1477–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenstein, J.; Roberts, S.B.; Dallal, G.; Saltzman, E. High-protein weight-loss diets: Are they safe and do they work? A review of the experimental and epidemiologic data. Nutr. Rev. 2002, 60, 189–200. [Google Scholar] [CrossRef]
- Soenen, S.; Westerterp-Plantenga, M.S. Proteins and satiety: Implications for weight management. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 747–751. [Google Scholar] [CrossRef] [Green Version]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Da Silva Lucas, A.J.; de Oliveira, L.M.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Womeni, H.M.; Linder, M.; Tiencheu, B.; Mbiapo, F.T.; Villeneuve, P.; Fanni, J.; Parmentier, M. Oils of insects and larvae consumed in Africa: Potential sources of polyunsaturated fatty acids. Oléagineux Corps Gras Lipides 2009, 16, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Christensen, D.L.; Orech, F.O.; Mungai, M.N.; Larsen, T.; Friis, H.; Aagaard-Hansen, J. Entomophagy among the Luo of Kenya: A potential mineral source? Int. J. Food Sci. Nutr. 2006, 57, 198–203. [Google Scholar] [CrossRef]
- Virginia, M.-R.; Tomás, Q.-B.; Rafael, D.-G.; César, G.-U. Consumption of Escamoles (Liometopum apiculatum M.): A Source of Vitamins A and E. J. Appl. Life Sci. Int. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover. Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheyen, G.R.; Pieters, L.; Maregesi, S.; Van Miert, S. Insects as Diet and Therapy: Perspectives on Their Use for Combating Diabetes Mellitus in Tanzania. Pharmaceuticals 2021, 14, 1273. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: A comparative survey and review. J. Ethnobiol. Ethnomed. 2017, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Neto, E.M. Entomotherapy, or the medicinal use of insects. J. Ethnobiol. 2005, 25, 93–114. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, ecological, safety and legislation aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef] [PubMed]
- Miguéns-Gómez, A.; Grau-Bové, C.; Sierra-Cruz, M.; Jorba-Martín, R.; Caro, A.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Blay, M.T.; Terra, X.; Ardévol, A.; et al. Gastrointestinally digested protein from the insect alphitobius diaperinus stimulates a different intestinal secretome than beef or almond, producing a differential response in food intake in rats. Nutrients 2020, 12, 2366. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbs, R.J.; Hughes, D.A.; Johnstone, A.M.; Rowley, E.; Reid, C.; Elia, M.; Stratton, R.; Delargy, H.; King, N.; Blundell, J.E. The use of visual analogue scales to assess motivation to eat in human subjects: A review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br. J. Nutr. 2000, 84, 405–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, Y.T.; Wu, J.S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. Methods 2018, 50, 1694–1715. [Google Scholar] [CrossRef] [PubMed]
- Cassady, B.A.; Hollis, J.H.; Fulford, A.D.; Considine, R.V.; Mattes, R.D. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. Am. J. Clin. Nutr. 2009, 89, 794–800. [Google Scholar] [CrossRef] [Green Version]
- House, J.D.; Hill, K.; Neufeld, J.; Franczyk, A.; Nosworthy, M.G. Determination of the protein quality of almonds ( Prunus dulcis L.) as assessed by in vitro and in vivo methodologies. Food Sci. Nutr. 2019, 7, 2932–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouhani, M.H.; Surkan, P.J.; Azadbakht, L. The effect of preload/meal energy density on energy intake in a subsequent meal: A systematic review and meta-analysis. Eat. Behav. 2017, 26, 6–15. [Google Scholar] [CrossRef]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In vitro crude protein digestibility of tenebrio molitor and hermetia illucens insect meals and its correlation with chemical composition traits. Ital. J. Anim. Sci. 2015, 14, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Hull, S.; Re, R.; Chambers, L.; Echaniz, A.; Wickham, M.S.J. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur. J. Nutr. 2015, 54, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chungchunlam, S.M.S.; Henare, S.J.; Ganesh, S.; Moughan, P.J. Effects of whey protein and its two major protein components on satiety and food intake in normal-weight women. Physiol. Behav. 2017, 175, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Borzoei, S.; Neovius, M.; Barkeling, B.; Teixeira-Pinto, A.; Rössner, S. A comparison of effects of fish and beef protein on satiety in normal weight men. Eur. J. Clin. Nutr. 2006, 60, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruijschop, R.M.A.J.; Burseg, K.M.M.; Lambers, T.T.; Overduin, J. Designing foods to induce satiation: A flavour perspective. In Designing Functional Foods: Measuring and Controlling Food Structure Breakdown and Nutrient Absorption; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 623–646. ISBN 9781845694326. [Google Scholar]
- Sørensen, L.B.; Møller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. 2003, 27, 1152–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Graaf, C.; De Jong, L.S.; Lambers, A.C. Palatability affects satiation but not satiety. Physiol. Behav. 1999, 66, 681–688. [Google Scholar] [CrossRef]
- Warwick, Z.S.; Hall, W.G.; Pappas, T.N.; Schiffman, S.S. Taste and smell sensations enhance the satiating effect of both a high-carbohydrate and a high-fat meal in humans. Physiol. Behav. 1993, 53, 553–563. [Google Scholar] [CrossRef]
- Rogers, P.J.; Blundell, J.E. Umami and appetite: Effects of monosodium glutamate on hunger and food intake in human subjects. Physiol. Behav. 1990, 48, 801–804. [Google Scholar] [CrossRef]
- Wells, A.S.; Read, N.W.; Uvnas-Moberg, K.; Alster, P. Influences of fat and carbohydrate on postprandial sleepiness, mood, and hormones. Physiol. Behav. 1997, 61, 679–686. [Google Scholar] [CrossRef]
- Hill, A.J.; Blundell, J.E. Macronutrients and satiety: The effects of a high-protein or high-carbohydrate meal on subjective motivation to eat and food preferences. Nutr. Behav. 1986, 3, 133–144. [Google Scholar]
- Barkeling, B.; Rossner, S.; Bjorvell, H. Effects of a high-protein meal (meat) and a high-carbohydrate meal (vegetarian) on satiety measured by automated computerized monitoring of subsequent food intake, motivation to eat and food preferences. Int. J. Obes. 1990, 14, 743–751. [Google Scholar] [PubMed]
- Griffioen-Roose, S.; Mars, M.; Finlayson, G.; Blundell, J.E.; De Graaf, C. The effect of within-meal protein content and taste on subsequent food choice and satiety. Br. J. Nutr. 2011, 106, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, J.; Mattes, R. Effect of chronic consumption of almonds on body weight in healthy humans. Br. J. Nutr. 2007, 98, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Mattes, R.D. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: A randomized, controlled trial. Eur. J. Clin. Nutr. 2013, 67, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. A nutrition and health perspective on almonds. Rev. Fitoter. 2013, 13, 125–135. [Google Scholar] [CrossRef]
- Hurd, K.J.; Shertukde, S.; Toia, T.; Trujillo, A.; Pérez, R.L.; Larom, D.L.; Love, J.J.; Liu, C. The Cultural Importance of Edible Insects in Oaxaca, Mexico. Ann. Entomol. Soc. Am. 2019, 112, 552–559. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. Are edible insects more or less “healthy” than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition. Eur. J. Clin. Nutr. 2016, 70, 285–291. [Google Scholar] [CrossRef] [PubMed]






| Preload | Vehicle | Almond | Insect |
|---|---|---|---|
| Nutritional analysis | |||
| Amount (g) | 145 | 205 | 170 |
| Energy (kJ) | 279.2 | 1854.2 | 812.2 |
| Fat (g) | 1 | 34 | 8.2 |
| Carbohydrate (g) | 7.7 | 10.3 | 8.4 |
| Fibre (g) | 8.6 | 0.9 | |
| Protein (g) | 5.7 | 20.1 | 20.7 |
| Content | |||
| Test food (g) | 60 | 25 | |
| Cocoa powder (g) | 5 | 5 | 5 |
| Sucralose (g) | 0.025 | 0.025 | 0.025 |
| Milk (g) | 140 | 140 | 140 |
| Food colourant (mL) | 0.5 | 0.5 | 0.5 |
| Preload Type | Vehicle | Insect | Almond |
|---|---|---|---|
| Overall likeability of preload meal | 7.98 ± 0.24 a | 5.34 ± 0.28 b | 5.36 ± 0.28 b |
| Pleasantness of taste | 7.61 ± 0.40 a | 5. 34 ± 0.49 b | 6.14 ± 0.39 a,b |
| Likeability of texture | 8.46 ± 0.38 a | 4.00 ± 0.45 b | 3.21 ± 0.45 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguéns-Gómez, A.; Sierra-Cruz, M.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Effect of an Acute Insect Preload vs. an Almond Preload on Energy Intake, Subjective Food Consumption and Intestinal Health in Healthy Young Adults. Nutrients 2022, 14, 1463. https://doi.org/10.3390/nu14071463
Miguéns-Gómez A, Sierra-Cruz M, Rodríguez-Gallego E, Beltrán-Debón R, Blay MT, Terra X, Pinent M, Ardévol A. Effect of an Acute Insect Preload vs. an Almond Preload on Energy Intake, Subjective Food Consumption and Intestinal Health in Healthy Young Adults. Nutrients. 2022; 14(7):1463. https://doi.org/10.3390/nu14071463
Chicago/Turabian StyleMiguéns-Gómez, Alba, Marta Sierra-Cruz, Esther Rodríguez-Gallego, Raúl Beltrán-Debón, M Teresa Blay, Ximena Terra, Montserrat Pinent, and Anna Ardévol. 2022. "Effect of an Acute Insect Preload vs. an Almond Preload on Energy Intake, Subjective Food Consumption and Intestinal Health in Healthy Young Adults" Nutrients 14, no. 7: 1463. https://doi.org/10.3390/nu14071463