How Alpha Linolenic Acid May Sustain Blood–Brain Barrier Integrity and Boost Brain Resilience against Alzheimer’s Disease
Abstract
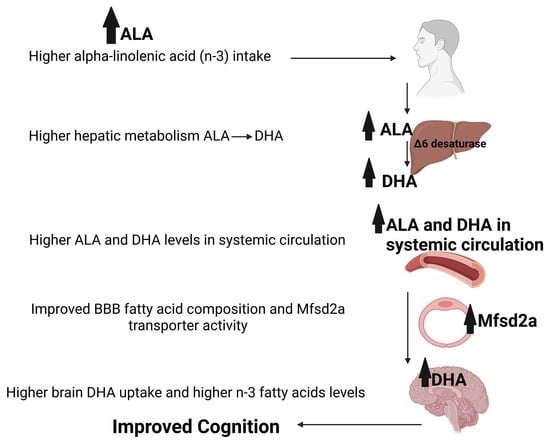
:1. Introduction
2. The Blood–Brain Barrier (BBB)
3. Lipids and BBB Function
4. BBB Breakdown in AD from a Lipid Perspective
5. Fatty Acids Role in Brain Health
6. Dietary Fatty Acids
7. Fatty Acid Metabolism
8. Lifespan Fatty Acid Modifications
9. Cholesterol
10. ApoE4 and BBB Lipids in AD
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montagne, A.; Nation, D.A.; Sagare, A.P.; Barisano, G.; Sweeney, M.D.; Chakhoyan, A.; Pachicano, M.; Joe, E.; Nelson, A.R.; D’Orazio, L.M.; et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature 2020, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zvi, A.; Lacoste, B.; Kur, E.; Andreone, B.J.; Mayshar, Y.; Yan, H.; Gu, C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 2014, 509, 507–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef] [Green Version]
- Rand, D.; Cooper, I. Caspase-1: An important player and possible target for repair of the blood-brain barrier underlying neurodegeneration. Neural Regen. Res. 2021, 16, 2390–2392. [Google Scholar]
- Hung, L.; Levine, H.; Randhawa, P.; Park, J. Technology-based group exercise interventions for people living with dementia or mild cognitive impairment: A scoping review protocol. BMJ Open 2022, 12, e055990. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement 2022, 8, e12295. [Google Scholar] [CrossRef]
- Lotan, R.; Ganmore, I.; Livny, A.; Itzhaki, N.; Waserman, M.; Shelly, S.; Zacharia, M.; Moshier, E.; Uribarri, J.; Beisswenger, P.; et al. Effect of Advanced Glycation End Products on Cognition in Older Adults with Type 2 Diabetes: Results from a Pilot Clinical Trial. J. Alzheimers Dis. 2021, 82, 1785–1795. [Google Scholar] [CrossRef]
- Hollander, K.S.; Tempel Brami, C.; Konikoff, F.M.; Fainaru, M.; Leikin-Frenkel, A. Dietary enrichment with alpha-linolenic acid during pregnancy attenuates insulin resistance in adult offspring in mice. Arch. Physiol. Biochem. 2014, 120, 99–111. [Google Scholar] [CrossRef]
- Shomonov-Wagner, L.; Raz, A.; Leikin-Frenkel, A. Alpha linolenic acid in maternal diet halts the lipid disarray due to saturated fatty acids in the liver of mice offspring at weaning. Lipids Health Dis. 2015, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Elhaik Goldman, S.; Goez, D.; Last, D.; Naor, S.; Liraz Zaltsman, S.; Sharvit-Ginon, I.; Atrakchi-Baranes, D.; Shemesh, C.; Twitto-Greenberg, R.; Tsach, S.; et al. High-fat diet protects the blood-brain barrier in an Alzheimer’s disease mouse model. Aging Cell 2018, 17, e12818. [Google Scholar] [CrossRef] [PubMed]
- Gille, B.; Galuska, C.E.; Fuchs, B.; Peleg, S. Recent Advances in Studying Age-Associated Lipids Alterations and Dietary Interventions in Mammals. Front. Aging 2021, 2, 773795. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2017, 15, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Bowman, G.L.; Dodge, H.H.; Guyonnet, S.; Zhou, N.; Donohue, J.; Bichsel, A.; Schmitt, J.; Hooper, C.; Bartfai, T.; Andrieu, S.; et al. A blood-based nutritional risk index explains cognitive enhancement and decline in the multidomain Alzheimer prevention trial. Alzheimers Dement 2019, 5, 953–963. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Saif, N.; Ariza, I.E.; Isaacson, R.S. Precision nutrition for alzheimer’s prevention in apoe4 carriers. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Yassine, H.N.; Samieri, C.; Livingston, G.; Glass, K.; Wagner, M.; Tangney, C.; Plassman, B.L.; Ikram, M.A.; Voigt, R.M.; Gu, Y.; et al. Nutrition state of science and dementia prevention: Recommendations of the Nutrition for Dementia Prevention Working Group. Lancet Healthy Longev. 2022, 3, e501–e512. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Epand, R.M. Membrane lipid polymorphism: Relationship to bilayer properties and protein function. Methods Mol. Biol. 2007, 400, 15–26. [Google Scholar]
- Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated fatty acids mediated regulation of membrane biochemistry and tumor cell membrane integrity. Membranes 2021, 11, 479. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, M.S.; Oliveros, L.B.; Gomez, N.N. Nutritional deficiencies and phospholipid metabolism. Int. J. Mol. Sci. 2011, 12, 2408–2433. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-lipid therapy: A new approach in molecular medicine. Trends Mol. Med. 2006, 12, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Leikin-Frenkel, A.; Liraz-Zaltsman, S.; Hollander, K.S.; Atrakchi, D.; Ravid, O.; Rand, D.; Kandel-Kfir, M.; Israelov, H.; Cohen, H.; Kamari, Y.; et al. Dietary alpha linolenic acid in pregnant mice and during weaning increases brain docosahexaenoic acid and improves recognition memory in the offspring. J. Nutr. Biochem. 2021, 91, 108597. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.-Y.; Ke, D.-S.; Chen, J.-Y. Essential fatty acids and human brain. Acta Neurol. Taiwan 2009, 18, 231–241. [Google Scholar] [PubMed]
- Bazan, N.G.; Musto, A.E.; Knott, E.J. Endogenous signaling by omega-3 docosahexaenoic acid-derived mediators sustains homeostatic synaptic and circuitry integrity. Mol. Neurobiol. 2011, 44, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Svennerholm, L.; Boström, K.; Jungbjer, B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 1997, 94, 345–352. [Google Scholar] [CrossRef]
- Eelen, G.; de Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.W.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [Green Version]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annu. Rev. Nutr. 2005, 25, 317–340. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Hennebelle, M.; Plourde, M.; Chouinard-Watkins, R.; Castellano, C.-A.; Barberger-Gateau, P.; Cunnane, S.C. Ageing and apoE change DHA homeostasis: Relevance to age-related cognitive decline. Proc. Nutr. Soc. 2014, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Walker, L.; Rauchmann, B.-S.; Perneczky, R. Dysfunction of the blood-brain barrier in Alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Cullis, P.R.; de Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.-F.; Wan, C.-Q.; Cai, M.; Zhou, N.-K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Barnes, S.; Chowdhury, S.; Gatto, N.M.; Fraser, G.E.; Lee, G.J. Omega-3 fatty acids are associated with blood-brain barrier integrity in a healthy aging population. Brain Behav. 2021, 11, e2273. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Kuan, Y.-H.; Li, J.-R.; Chen, W.-Y.; Ou, Y.-C.; Pan, H.-C.; Liao, S.-L.; Raung, S.-L.; Chang, C.-J.; Chen, C.-J. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef]
- Kuo, Y.-T.; So, P.-W.; Parkinson, J.R.; Yu, W.S.; Hankir, M.; Herlihy, A.H.; Goldstone, A.P.; Frost, G.S.; Wasserfall, C.; Bell, J.D. The combined effects on neuronal activation and blood-brain barrier permeability of time and n-3 polyunsaturated fatty acids in mice, as measured in vivo using MEMRI. Neuroimage 2010, 50, 1384–1391. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Pasker-de Jong, P.C.M.; de Vries, R.B.M.; Ritskes-Hoitinga, M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer’s pathology in animal models of Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2012, 28, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, Y.; Shinohara, M.; Shinohara, M.; Yamazaki, A.; Murray, M.E.; Liesinger, A.M.; Heckman, M.G.; Lesser, E.R.; Parisi, J.E.; Petersen, R.C.; et al. Selective loss of cortical endothelial tight junction proteins during Alzheimer’s disease progression. Brain 2019, 142, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Bené, H. Polyunsaturated fatty acid regulation of gene expression. J. Mol. Neurosci. 2001, 16, 273–278, discussion 279. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.P.; Nakamura, M.; Clarke, S.D. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J. Biol. Chem. 1999, 274, 37335–37339. [Google Scholar] [CrossRef] [Green Version]
- McNamara, R.K.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P. The aging human orbitofrontal cortex: Decreasing polyunsaturated fatty acid composition and associated increases in lipogenic gene expression and stearoyl-CoA desaturase activity. Prostaglandins Leukot Essent Fatty Acids 2008, 78, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazan, N.G. Synaptic lipid signaling: Significance of polyunsaturated fatty acids and platelet-activating factor. J. Lipid Res. 2003, 44, 2221–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636. [Google Scholar] [CrossRef]
- Wood, C.A.P.; Zhang, J.; Aydin, D.; Xu, Y.; Andreone, B.J.; Langen, U.H.; Dror, R.O.; Gu, C.; Feng, L. Structure and mechanism of blood-brain-barrier lipid transporter MFSD2A. Nature 2021, 596, 444–448. [Google Scholar] [CrossRef]
- Leikin, A.; Shinitzky, M. Characterization of the lipid surrounding the delta 6-desaturase of rat liver microsomes. Biochim. Biophys. Acta 1995, 1256, 13–17. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael-Titus, A.T. Neurological benefits of omega-3 fatty acids. Neuromolecul. Med. 2008, 10, 219–235. [Google Scholar] [CrossRef]
- Von Schacky, C. Importance of EPA and DHA blood levels in brain structure and function. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Liu, J.-H.; Wang, Q.; You, Q.-L.; Li, Z.-L.; Hu, N.-Y.; Wang, Y.; Jin, Z.-L.; Li, S.-J.; Li, X.-W.; Yang, J.-M.; et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020, 11, 5465. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Martin, J.-C.; Agnani, G.; Pages, N.; Leruyet, P.; Carayon, P.; Delplanque, B. Dairy fat blends high in α-linolenic acid are superior to n-3 fatty-acid-enriched palm oil blends for increasing DHA levels in the brains of young rats. J. Nutr. Biochem. 2012, 23, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.C.; Cook-Johnson, R.J.; James, M.J.; Mühlhäusler, B.S.; Gibson, R.A. Omega-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins Leukot Essent Fatty Acids 2010, 83, 61–68. [Google Scholar] [CrossRef]
- Majou, D. Synthesis of DHA (omega-3 fatty acid): FADS2 gene polymorphisms and regulation by PPARα. OCL 2021, 28, 43. [Google Scholar] [CrossRef]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Lindenau, K.L.; Barr, J.L.; Higgins, C.R.; Sporici, K.T.; Brailoiu, E.; Brailoiu, G.C. Blood-Brain Barrier Disruption Mediated by FFA1 Receptor-Evidence Using Miniscope. Int. J. Mol. Sci. 2022, 23, 2258. [Google Scholar] [CrossRef]
- Heath, R.J.; Wood, T.R. Why have the benefits of DHA not been borne out in the treatment and prevention of alzheimer’s disease? A narrative review focused on DHA metabolism and adipose tissue. Int. J. Mol. Sci. 2021, 22, 1826. [Google Scholar] [CrossRef]
- Brenner, R.R. The oxidative desaturation of unsaturated fatty acids in animals. Mol. Cell. Biochem. 1974, 3, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.-M.; Vasilevko, V.; Dipatrizio, N.V.; Martin, S.K.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS ONE 2011, 6, e24777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leikin-Frenkel, A.; Cohen, H.; Keshet, R.; Shnerb-GanOr, R.; Kandel-Kfir, M.; Harari, A.; Hollander, K.S.; Shaish, A.; Harats, D.; Kamari, Y. The effect of α-linolenic acid enrichment in perinatal diets in preventing high fat diet-induced SCD1 increased activity and lipid disarray in adult offspring of low density lipoprotein receptor knockout (LDLRKO) mice. Prostaglandins Leukot. Essent. Fatty Acids 2022, 184, 102475. [Google Scholar] [CrossRef] [PubMed]
- Bernoud, N.; Fenart, L.; Bénistant, C.; Pageaux, J.F.; Dehouck, M.P.; Molière, P.; Lagarde, M.; Cecchelli, R.; Lecerf, J. Astrocytes are mainly responsible for the polyunsaturated fatty acid enrichment in blood-brain barrier endothelial cells in vitro. J. Lipid Res. 1998, 39, 1816–1824. [Google Scholar] [CrossRef]
- Taylor, X.; Cisternas, P.; Jury, N.; Martinez, P.; Huang, X.; You, Y.; Redding-Ochoa, J.; Vidal, R.; Zhang, J.; Troncoso, J.; et al. Activated endothelial cells induce a distinct type of astrocytic reactivity. Commun. Biol. 2022, 5, 282. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zang, G.; Zhang, T.; Wu, Q.; Zhang, H.; Chen, Y.; Wang, Y.; Qin, W.; Zhao, S.; et al. trans-2-Enoyl-CoA Reductase Tecr-Driven Lipid Metabolism in Endothelial Cells Protects against Transcytosis to Maintain Blood-Brain Barrier Homeostasis. Research 2022, 2022, 9839368. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal dietary polyunsaturated fatty acids in brain development, role in neurodevelopmental disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16, 4600. [Google Scholar] [CrossRef] [Green Version]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Burdge, G. Alpha-linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Bordoni, A.; Celadon, M.; Turchetto, E.; Biagi, P.L.; Rossi, C.A. Age-related changes in linoleate and alpha-linolenate desaturation by rat liver microsomes. Biochem. Biophys. Res. Commun. 1989, 163, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riddell, D.R.; Zhou, H.; Atchison, K.; Warwick, H.K.; Atkinson, P.J.; Jefferson, J.; Xu, L.; Aschmies, S.; Kirksey, Y.; Hu, Y.; et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J. Neurosci. 2008, 28, 11445–11453. [Google Scholar] [CrossRef] [Green Version]
- Varma, V.R.; Büşra Lüleci, H.; Oommen, A.M.; Varma, S.; Blackshear, C.T.; Griswold, M.E.; An, Y.; Roberts, J.A.; O’Brien, R.; Pletnikova, O.; et al. Abnormal brain cholesterol homeostasis in Alzheimer’s disease-a targeted metabolomic and transcriptomic study. Npj Aging Mech. Dis. 2021, 7, 11. [Google Scholar] [CrossRef]
- Liu, C.-C.; Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [Green Version]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Vandal, M.; Alata, W.; Tremblay, C.; Rioux-Perreault, C.; Salem, N.; Calon, F.; Plourde, M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 2014, 129, 516–526. [Google Scholar] [CrossRef]
- Barisano, G.; Kisler, K.; Wilkinson, B.; Nikolakopoulou, A.M.; Sagare, A.P.; Wang, Y.; Gilliam, W.; Huuskonen, M.T.; Hung, S.-T.; Ichida, J.K.; et al. A “multi-omics” analysis of blood-brain barrier and synaptic dysfunction in APOE4 mice. J. Exp. Med. 2022, 219, e20221137. [Google Scholar] [CrossRef]
- Saidi, S.; Slamia, L.B.; Ammou, S.B.; Mahjoub, T.; Almawi, W.Y. Association of apolipoprotein E gene polymorphism with ischemic stroke involving large-vessel disease and its relation to serum lipid levels. J. Stroke Cerebrovasc. Dis. 2007, 16, 160–166. [Google Scholar] [CrossRef]
- Miranda, A.M.; Ashok, A.; Chan, R.B.; Zhou, B.; Xu, Y.; McIntire, L.B.; Area-Gomez, E.; Di Paolo, G.; Duff, K.E.; Oliveira, T.G.; et al. Effects of APOE4 allelic dosage on lipidomic signatures in the entorhinal cortex of aged mice. Transl. Psychiatry 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Shi, X.; Gu, H.; Brinton, R.D.; Yin, F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021, 34, 108572. [Google Scholar] [CrossRef] [PubMed]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, F. Lipid metabolism and Alzheimer’s disease: Clinical evidence, mechanistic link and therapeutic promise. FEBS J. 2022, 7. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leikin-Frenkel, A.; Schnaider Beeri, M.; Cooper, I. How Alpha Linolenic Acid May Sustain Blood–Brain Barrier Integrity and Boost Brain Resilience against Alzheimer’s Disease. Nutrients 2022, 14, 5091. https://doi.org/10.3390/nu14235091
Leikin-Frenkel A, Schnaider Beeri M, Cooper I. How Alpha Linolenic Acid May Sustain Blood–Brain Barrier Integrity and Boost Brain Resilience against Alzheimer’s Disease. Nutrients. 2022; 14(23):5091. https://doi.org/10.3390/nu14235091
Chicago/Turabian StyleLeikin-Frenkel, Alicia, Michal Schnaider Beeri, and Itzik Cooper. 2022. "How Alpha Linolenic Acid May Sustain Blood–Brain Barrier Integrity and Boost Brain Resilience against Alzheimer’s Disease" Nutrients 14, no. 23: 5091. https://doi.org/10.3390/nu14235091