Moderate Hyperglycemia-Preventive Effect and Mechanism of Action of Periplaneta americana Oligosaccharides in Streptozotocin-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of PAOS
2.3. Animals
2.4. Establishment of the DM Model and PAOS Treatment
2.5. Fasting Serum Insulin Levels
2.6. Measurement of Cytokines, Serum Parameters and Organ Indexes
2.7. Measurement of Liver Function and Antioxidant Parameters
2.8. Immunohistochemistry Staining
2.9. Western Blot
2.10. Microbial Community Analysis
2.11. Statistical Analysis
3. Results
3.1. Effects of PAOS on DM Were Revealed by Body Weight, FBG, Insulin and HOMA-β
3.2. PAOS Regulated Immune Responses in DM Mice
3.3. PAOS Reduced Serum Parameterslevels
3.4. PAOS Benefited Liver Function and Antioxidant Parameters in DM Mice
3.5. PAOS Improved Islet β Cell Function and Downregulated Its Apoptosis in DM Mice
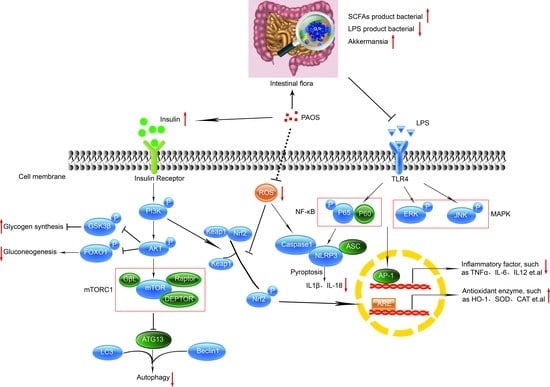
3.6. PAOS Regulated Signal Pathways in the Livers of TIDM Mice
3.7. PAOS Regulated the Gut Microbiota in Control Mice
3.8. PAOS Ameliorated on Gut Microbiota in DM Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Satija, A.; Manson, J.E. Curbing the Diabetes Pandemic: The Need for Global Policy Solutions. JAMA 2015, 313, 2319–2320. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas. In Idf Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Kerru, N.; Singh-Pillay, A.; Awolade, P.; Singh, P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018, 152, 436–488. [Google Scholar] [CrossRef]
- Buzzetti, R.; Zampetti, S.; Maddaloni, E. Adult-onset autoimmune diabetes: Current knowledge and implications for management. Nat. Rev. Endocrinol. 2017, 13, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Reddivari, L.; Shi, Z.; Li, S.; Wang, Y.; Bretin, A.; Ngo, V.L.; Flythe, M.; Pellizzon, M.; Chassaing, B.; et al. Inulin Fermentable Fiber Ameliorates Type I Diabetes via IL22 and Short-Chain Fatty Acids in Experimental Models. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 983–1000. [Google Scholar] [CrossRef]
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, A.; Tu, P.; Hu, Z. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin. Med. 2017, 12, 26. [Google Scholar] [CrossRef]
- Nguyen, T.; Chen, X.; Chai, J.; Li, R.; Han, X.; Chen, X.; Liu, S.; Chen, M.; Xu, X. Antipyretic, anti-inflammatory and analgesic activities of Periplaneta americana extract and underlying mechanisms. Biomed. Pharmacother. 2020, 123, 109753. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Liu, J.; Wu, X.; Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus? FASEB J. 2019, 33, 11655–11667. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.M.; Zhou, J.; Deng, J.; Li, Y.J.; Wu, C.F.; Bao, J.K. Periplaneta americana Oligosaccharides Exert Anti-Inflammatory Activity through Immunoregulation and Modulation of Gut Microbiota in Acute Colitis Mice Model. Molecules 2021, 26, 1718. [Google Scholar] [CrossRef]
- Dhuria, R.S.; Singh, G.; Kaur, A.; Kaur, R.; Kaur, T. Current status and patent prospective of animal models in diabetic research. Adv. Biomed. Res. 2015, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Li, E.; Sullivan, M.A.; Tan, X.; Deng, B.; Gilbert, R.G.; Li, C. Glycogen structure in type 1 diabetic mice: Towards understanding the origin of diabetic glycogen molecular fragility. Int. J. Biol. Macromol. 2019, 128, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Veeranjaneyulu, A. Herbs for Diabetes and Neurological Disease Management; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Cani, P.D.; Knauf, C.; Iglesias, M.A.; Drucker, D.J.; Delzenne, N.M.; Burcelin, R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 2006, 55, 1484–1490. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Li, H.; Lu, Z.; Shi, J.; Xu, Z. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 2018, 9, 3916–3929. [Google Scholar] [CrossRef]
- Katiyar, D.; Singh, B.; Lall, A.M.; Haldar, C. Efficacy of chitooligosaccharides for the management of diabetes in alloxan induced mice: A correlative study with antihyperlipidemic and antioxidative activity. Eur. J. Pharm. Sci. 2011, 44, 534–543. [Google Scholar] [CrossRef]
- Ortega-González, M.; Ocón, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suárez, M.D.; de Medina, F.S.; Martínez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Li, Y.Y.; Zeng, H.X.; Liu, X.Q.; Sun, Y.T.; Jiang, L.; Xia, L.L.; Wu, Y.G. Carnosine alleviates podocyte injury in diabetic nephropathy by targeting caspase-1-mediated pyroptosis. Int. Immunopharmacol. 2021, 101, 108236. [Google Scholar] [CrossRef]
- Cobelli, C.; Toffolo, G.M.; Man, C.D.; Campioni, M.; Denti, P.; Caumo, A.; Butler, P.; Rizza, R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1–E15. [Google Scholar] [CrossRef]
- Obafemi, T.O.; Olaleye, M.T.; Akinmoladun, A.C. Antidiabetic property of miracle fruit plant (Synsepalum dulcificum Shumach. & Thonn. Daniell) leaf extracts in fructose-fed streptozotocin-injected rats via anti-inflammatory activity and inhibition of carbohydrate metabolizing enzymes. J. Ethnopharmacol. 2019, 244, 112124. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, P.; Gargari, B.P.; Jafar-abadi, M.A. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition 2014, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.; Arneth, R.; Shams, M. Metabolomics of Type 1 and Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Li, L.; Wu, J.; Gong, B.; Liu, H. Germacrone cooperates with dexmedetomidine to alleviate high-fat diet-induced type 2 diabetes mellitus via upregulating AMPKα1 expression. Exp. Ther. Med. 2019, 18, 3514–3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, N.; Morino, K.; Ida, S.; Sekine, O.; Lemecha, M.; Kume, S.; Park, S.-Y.; Choi, C.S.; Ugi, S.; Maegawa, H. Pivotal Role of O-GlcNAc Modification in Cold-Induced Thermogenesis by Brown Adipose Tissue Through Mitochondrial Biogenesis. Diabetes 2017, 66, 2351–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, R.; Minj, P.; Malik, N.; Sardesai, D.M.; Kulkarni, S.H.; Acharya, J.D.; Bhavesh, N.S.; Sharma, S.; Kumar, A. Recombinant human islet amyloid polypeptide forms shorter fibrils and mediates β-cell apoptosis via generation of oxidative stress. Biochem. J. 2017, 474, 3915–3934. [Google Scholar] [CrossRef]
- Ige, A.O.; Adewoye, E.O. Oral Magnesium Treatment Reduces Anemia and Levels of Inflammatory Markers in Experimental Diabetes. J. Diet. Suppl. 2017, 14, 76–88. [Google Scholar] [CrossRef]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.; Singh, A.K. Hypoglycaemic effect of galactooligosaccharides in alloxan-induced diabetic rats. J. Dairy Res. 2015, 82, 70–77. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. Mechanisms in endocrinology: Gut microbiota in patients with type 2 diabetes mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Li, Y.; Fang, J.; Liu, G.; Yin, J.; Li, T.; Yin, Y. Gut Microbiota and Type 1 Diabetes. Int. J. Mol. Sci. 2018, 19, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Vatanen, T.; Droit, L.; Park, A.; Kostic, A.D.; Poon, T.W.; Vlamakis, H.; Siljander, H.; Härkönen, T.; Hämäläinen, A.-M.; et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. USA 2017, 114, E6166–E6175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; He, Y.; Wu, C.; Bao, J. Moderate Hyperglycemia-Preventive Effect and Mechanism of Action of Periplaneta americana Oligosaccharides in Streptozotocin-Induced Diabetic Mice. Nutrients 2022, 14, 4620. https://doi.org/10.3390/nu14214620
Lu K, He Y, Wu C, Bao J. Moderate Hyperglycemia-Preventive Effect and Mechanism of Action of Periplaneta americana Oligosaccharides in Streptozotocin-Induced Diabetic Mice. Nutrients. 2022; 14(21):4620. https://doi.org/10.3390/nu14214620
Chicago/Turabian StyleLu, Kaimin, Yufei He, Chuanfang Wu, and Jinku Bao. 2022. "Moderate Hyperglycemia-Preventive Effect and Mechanism of Action of Periplaneta americana Oligosaccharides in Streptozotocin-Induced Diabetic Mice" Nutrients 14, no. 21: 4620. https://doi.org/10.3390/nu14214620