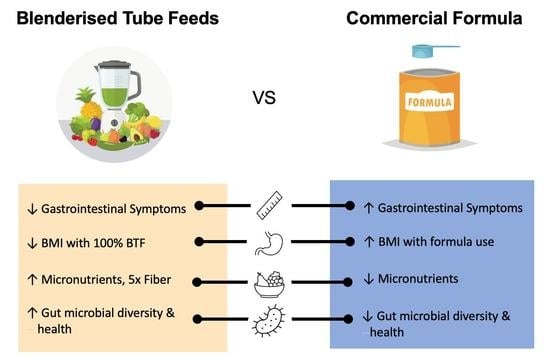
Blenderised Tube Feeds vs. Commercial Formula: Which Is Better for Gastrostomy-Fed Children?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. GI Peds-QL Questionnaire Scores
3.3. Anthropometrics
3.4. Nutritional Biochemistry
3.5. Nutritional Analysis of Feeds
3.6. Stool Sample Analysis
3.7. Parental Satisfaction with Feeding Regimen
4. Discussion
4.1. Gastrointestinal Symptoms
4.2. Growth
4.3. Nutrition
4.4. Stool Microbiota
4.5. Caregiver Experiences
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blumenstein, I.; Shastri, Y.M.; Stein, J. Gastroenteric tube feeding: Techniques, problems and solutions. World J. Gastroenterol. 2014, 20, 8505–8524. [Google Scholar] [CrossRef]
- Gramlich, L.; Hurt, R.T.; Jin, J.; Mundi, M.S. Home Enteral Nutrition: Towards a Standard of Care. Nutrients 2018, 10, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krom, H.; van Zundert, S.M.; Otten, M.A.G.; van der Sluijs Veer, L.; Benninga, M.A.; Kindermann, A. Prevalence and side effects of pediatric home tube feeding. Clin. Nutr. 2019, 38, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Wanden-Berghe, C.; Patino-Alonso, M.-C.; Galindo-Villardón, P.; Sanz-Valero, J. Complications Associated with Enteral Nutrition: CAFANE Study. Nutrients 2019, 11, 2041. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, K.; Flint, A.; Mouzaki, M.; Carpenter, A.; Haliburton, B.; Bannister, L.; Norgrove, H.; Hoffman, L.; Mack, D.; Stintzi, A.; et al. Blenderized Enteral Nutrition Diet Study: Feasibility, Clinical, and Microbiome Outcomes of Providing Blenderized Feeds Through a Gastric Tube in a Medically Complex Pediatric Population. J. Parenter. Enter. Nutr. 2018, 42, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Savino, P. Knowledge of Constituent Ingredients in Enteral Nutrition Formulas Can Make a Difference in Patient Response to Enteral Feeding. Nutr. Clin. Pract. 2018, 33, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hron, B.; Fishman, E.; Lurie, M.; Clarke, T.; Chin, Z.; Hester, L.; Burch, E.; Rosen, R. Health Outcomes and Quality of Life Indices of Children Receiving Blenderized Feeds via Enteral Tube. J. Pediatrics 2019, 211, 139–145.e131. [Google Scholar] [CrossRef] [PubMed]
- Trollip, A.; Lindeback, R.; Banerjee, K. Parental Perspectives on Blenderized Tube Feeds for Children Requiring Supplemental Nutrition. Nutr. Clin. Pract. 2020, 35, 471–478. [Google Scholar] [CrossRef]
- Craig, G.M. Psychosocial aspects of feeding children with neurodisability. Eur. J. Clin. Nutr. 2013, 67 (Suppl. S2), S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Oparaji, J.A.; Sferra, T.; Sankararaman, S. Basics of Blenderized Tube Feeds: A Primer for Pediatric Primary Care Clinicians. Gastroenterol. Res. 2019, 12, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillanders, L.; Angstmann, K.; Ball, P.; Chapman-Kiddell, C.; Hardy, G.; Hope, J.; Smith, R.; Strauss, B.; Russell, D. AuSPEN clinical practice guideline for home parenteral nutrition patients in Australia and New Zealand. Nutrition 2008, 24, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.; Buchanan, E.; Duncan, H.; Ross, K.; Gerasimidis, K. Dietitians’ perceptions and experience of blenderised feeds for paediatric tube-feeding. Arch. Dis. Child. 2017, 102, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.W.; Spurlock, A.; Pierce, L. Survey Study Assessing Attitudes and Experiences of Pediatric Registered Dietitians Regarding Blended Food by Gastrostomy Tube Feeding. Nutr. Clin. Pract. 2015, 30, 402–405. [Google Scholar] [CrossRef]
- Carter, H.; Johnson, K.; Johnson, T.W.; Spurlock, A. Blended tube feeding prevalence, efficacy, and safety: What does the literature say? J. Am. Assoc. Nurse Pract. 2018, 30, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Spurlock, A.L.; Epp, L.; Hurt, R.T.; Mundi, M.S. Reemergence of Blended Tube Feeding and Parent’s Reported Experiences in Their Tube Fed Children. J. Altern. Complement. Med. 2018, 24, 369–373. [Google Scholar] [CrossRef]
- Epp, L.; Lammert, L.; Vallumsetla, N.; Hurt, R.T.; Mundi, M.S. Use of Blenderized Tube Feeding in Adult and Pediatric Home Enteral Nutrition Patients. Nutr. Clin. Pract. 2017, 32, 201–205. [Google Scholar] [CrossRef]
- Batsis, I.D.; Davis, L.; Prichett, L.; Wu, L.; Shores, D.; Au Yeung, K.; Oliva-Hemker, M. Efficacy and Tolerance of Blended Diets in Children Receiving Gastrostomy Feeds. Nutr. Clin. Pract. 2020, 35, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Pentiuk, S.; O’Flaherty, T.; Santoro, K.; Willging, P.; Kaul, A. Pureed by gastrostomy tube diet improves gagging and retching in children with fundoplication. J. Parenter. Enter. Nutr. 2011, 35, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Samela, K.; Mokha, J.; Emerick, K.; Davidovics, Z.H. Transition to a Tube Feeding Formula With Real Food Ingredients in Pediatric Patients With Intestinal Failure. Nutr. Clin. Pract. 2017, 32, 277–281. [Google Scholar] [CrossRef]
- Varni, J.W.; Bendo, C.B.; Denham, J.; Shulman, R.J.; Self, M.M.; Neigut, D.A.; Nurko, S.; Patel, A.S.; Franciosi, J.P.; Saps, M.; et al. PedsQL Gastrointestinal Symptoms Module: Feasibility, Reliability, and Validity. J. Pediatric Gastroenterol. Nutr. 2014, 59, 347–355. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Including Recommended Dietary Intakes; Australian Government Department of Health and Ageing: Canberra, Australia; New Zealand Ministry of Health, National Health and Medical Research Council: Wellington, New Zealand, 2006. [Google Scholar]
- Pahsini, K.; Marinschek, S.; Khan, Z.; Dunitz-Scheer, M.; Scheer, P.J. Unintended Adverse Effects of Enteral Nutrition Support: Parental Perspective. J. Pediatric Gastroenterol. Nutr. 2016, 62, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Vieira, M.M.C.; Santos, V.F.N.; Bottoni, A.; Morais, T.B. Nutritional and microbiological quality of commercial and homemade blenderized whole food enteral diets for home-based enteral nutritional therapy in adults. Clin. Nutr. 2018, 37, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.F.; Morais, T.B. Nutritional quality and osmolality of home-made enteral diets, and follow-up of growth of severely disabled children receiving home enteral nutrition therapy. J. Trop. Pediatrics 2010, 56, 127–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orel, A.; Homan, M.; Blagus, R.; Benedik, E.; Orel, R.; Fidler Mis, N. Nutrition of Patients with Severe Neurologic Impairment. Radiol. Oncol. 2017, 52, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Novak, P.; Wilson, K.E.; Ausderau, K.; Cullinane, D. The use of blenderized tube feedings. ICAN Infant Child Adolesc. Nutr. 2009, 1, 21–23. [Google Scholar] [CrossRef]
- Sullivan, M.M.; Sorreda-Esguerra, P.; Platon, M.B.; Castro, C.G.; Chou, N.R.; Shott, S.; Corner, G.M.; Alarcon, P. Nutritional analysis of blenderized enteral diets in the Philippines. Asia Pac. J. Clin. Nutr. 2004, 13, 385–391. [Google Scholar]
- Tanchoco, C.C.; Castro, C.A.M.; Villadolid, M.F.; Casiño, G.; Rodriguez, M.P.; Roa, C.; Cruz, C.M.A.D.L.; Tangcongco, F., Jr. Enteral feeding in stable chronic obstructive pulmonary disease patients. Respirology 2001, 6, 43–50. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Bahramian, B.; Sarabi-Jamab, M.; Talebi, S.; Razavi, S.M.A.; Rezaie, M. Designing blenderized tube feeding diets for children and investigating their physicochemical and microbial properties and Dietary Inflammatory Index. Nutr Clin Pract. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Baird, P.; Davis, R.H.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brekke, G.; Raun, A.; Sørensen, S.B.; Kok, K.; Sørensen, J.L.; Born, A.P.; Mølgaard, C.; Hoei-Hansen, C.E. Nutrition and preparation of blenderized tube feeding in children and adolescents with neurological impairment: A scoping review. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2022, 37, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Morrison, M.; Cao, K.L.; Pruilh, S.; Davies, P.; Wall, C.; Lovell, A.; Hill, R.J. Dietary intake influences gut microbiota development of healthy Australian children from the age of one to two years. Sci. Rep. 2019, 9, 12476. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Jeffry, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Soscia, J.; Adams, S.; Cohen, E.; Moore, C.; Friedman, J.N.; Gallagher, K.; Marcon, M.; Nicholas, D.; Weiser, N.; Orkin, J. The parental experience and perceptions of blenderized tube feeding for children with medical complexity. Paediatr. Child Health 2021, 26, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, N.; Dehlsen, K.; Leach, S.T.; Krishnan, U. Exploring Clinical Outcomes and Feasibility of Blended Tube Feeds in Children. JPEN J. Parenter. Enter. Nutr. 2021, 45, 685–698. [Google Scholar] [CrossRef]
- Sullivan, P.B.; Juszczak, E.; Bachlet, A.M.; Thomas, A.G.; Lambert, B.; Vernon-Roberts, A.; Grant, H.W.; Eltumi, M.; Alder, N.; Jenkinson, C. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev. Med. Child Neurol. 2004, 46, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Wilken, M. The impact of child tube feeding on maternal emotional state and identity: A qualitative meta-analysis. J. Pediatr. Nurs. 2012, 27, 248–255. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | BTF (n = 20) | CF (n = 21) | p Value |
|---|---|---|---|
| Age (years) | 7.42 ± 4.24 | 6.54 ± 3.05 | 0.672 |
| Male | 13 (65%) | 13 (61.9%) | 0.990 |
| Time since G-Tube Insertion (years) 1 | 5.194 ± 3.438 | 4.095 ± 2.370 | 0.368 |
| Underlying Comorbidity | |||
| Neurological Impairment | 15 (71.43%) | 10 (50%) | 0.215 |
| Gastrointestinal Disease 2 | 9 (42.86%) | 4 (20%) | 0.186 |
| Cystic Fibrosis | 0 (0%) | 2 (10%) | 0.233 |
| OA-TOF 3 | 1 (4.76%) | 2 (10%) | 0.614 |
| Other | 2 (9.52%) | 3 (15%) | 0.669 |
| Feeding Method | |||
| Continuous | 0% | 6 (30%) | 0.009 * |
| Bolus | 13 (61.90%) | 7 (35.00) | 0.124 |
| Mixed | 8 (38.10%) | 7 (23.33%) | 0.351 |
| Oral | 12 (57.14%) | 7 (35.00%) | 0.215 |
| Medications | |||
| PPI 4 | 8 (38.1%) | 12 (60%) | 0.211 |
| Pro-Kinetics | 2 (9.52%) | 8 (40%) | 0.037 * |
| Stool Softener/Laxatives | 2 (9.52%) | 7 (35%) | 0.072 |
| Anti-Diarrhoeal | 2 (9.52%) | 1 (5%) | >0.999 |
| Average Duration of Time on BTF (years) | |||
| 2.24 | |||
| % Caloric Intake provided by BTF | |||
| 25–50% | 4 (20%) | ||
| 50% | 9 (45%) | ||
| 100% | 7 (35%) | ||
| Peds-QL Section | BTF (n = 20) | CF (n = 18) | p Value |
|---|---|---|---|
| Stomach Pain | 78.24 ± 16.63 | 58.10 ± 24.45 | 0.0051 * |
| Stomach Upset | 83.89 ± 15.84 | 54.44 ± 29.55 | 0.0004 * |
| Food and Drink Limits | 47.22± 43.84 | 24.07 ± 35.66 | 0.0952 * |
| Trouble Swallowing | 36.11 ± 36.25 | 29.17 ± 27.63 | 0.5271 |
| Heartburn/Reflux | 77.43 ± 16.93 | 50.00 ± 29.67 | 0.0016 * |
| Nausea/Vomiting | 81.60 ± 21.54 | 36.11 ± 22.11 | <0.0001 * |
| Gas | 70.83 ± 18.15 | 47.69 ± 24.70 | 0.0027 * |
| Constipation | 79.70 ± 19.57 | 49.60 ± 27.90 | 0.0003 * |
| Blood in Stool | 98.61 ± 5.735 | 70.31 ± 39.49 | 0.0046 * |
| Diarrhoea | 83.93 ± 20.92 | 64.18 ± 23.67 | 0.0282 * |
| Total GI Symptoms 1 | 73.76 ± 10.41 | 48.61 ± 17.16 | <0.0001 * |
| Worry about Stool | 89.23 ± 89.23 (n = 13) | 59.07 ± 37.07 (n = 9) | 0.0200 * |
| Worry about Abdominal Pain | 89.58 ± 19.82 (n = 12) | 53.13 ± 36.44 (n = 8) | 0.0095 * |
| Medications | 93.33 ± 12.38 (n = 15) | 70.54 ± 40.30 (n = 7) | 0.0545 |
| Communication | 42.33 ± 39.95 (n = 15) | 39.38 ± 35.60 (n = 8) | 0.8625 |
| Total Score | 74.65 ± 8.136 (n = 12) | 50.125 ± 27.44 (n = 7) | 0.004 * |
| % BTF Groups | Mean Difference | Mean 1 | Mean 1 | Standard Error | p Value | Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| CF vs. 25 | −23.20 | 51.26 | 74.46 | 7.786 | 0.0553 | −41.51 | 0.7520 |
| CF vs. 50 | −23.85 | 51.26 | 75.11 | 5.804 | 0.0012 * | −40.19 | −8.692 |
| CF vs. 100 | −20.12 | 51.26 | 71.38 | 6.668 | 0.0227 * | −38.81 | −2.619 |
| 25 vs. 50 | −0.6488 | 74.46 | 75.11 | 8.370 | 0.9999 | −26.78 | 18.65 |
| 25 vs. 100 | 3.079 | 74.46 | 71.38 | 8.991 | 0.9891 | −24.74 | 24.07 |
| 50 vs. 100 | 3.728 | 75.11 | 71.38 | 7.341 | 0.9566 | −16.20 | 23.65 |
| Characteristics | BTF (n = 20) | CF (n = 21) | p Value |
|---|---|---|---|
| Height Z-Score | −1.239 ± 2.419 (n = 17) | −1.660 ± 1.047 | 0.905 |
| Weight Z-Score | −1.722 ± 2.140 | −0.506 ± 1.38 | 0.0108 * |
| BMI Z-Score | −0.597 ± 2.099 (n = 17) | 0.428 ± 0.8455 | 0.045 * |
| % Malnourished | 4 (20%) | 1 (4.76%) | 0.183 |
| % BTF Groups | Mean Difference | Mean 1 | Mean 1 | Standard Error | p Value | Confidence Interval | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| CF vs. 25 | 0.5905 | 0.4280 | −0.1625 | 0.6796 | 0.8207 | −0.6856 | 2.610 |
| CF vs. 50 | −0.04771 | 0.4280 | 0.4757 | 0.5449 | 0.9998 | −1.395 | 1.504 |
| CF vs. 100 | 3.361 | 0.4280 | −2.933 | 0.6796 | <0.0001 * | 1.659 | 5.266 |
| 25 vs. 50 | −0.6382 | −0.1625 | 0.4757 | 0.7777 | 0.8442 | −2.827 | 1.012 |
| 25 vs. 100 | 2.770 | −0.1625 | −2.933 | 0.8773 | 0.0176 * | 0.3016 | 4.699 |
| 50 vs. 100 | 3.408 | 0.4757 | −2.933 | 0.7777 | 0.0007 * | 1.354 | 5.463 |
| Component | BTF | CF | p Value |
|---|---|---|---|
| Albumin | 38.12 ± 4.512 (n = 17) | 41.86 ± 22.81 (n = 14) | 0.7908 |
| Corrected Calcium | 2.398 ± 0.111 (n = 16) | 2.356 ± 0.0829 (n = 13) | 0.2691 |
| Ferritin | 85.00 ± 157.3 (n = 15) | 51.30 ± 41.66 (n = 12) | 0.5451 |
| Magnesium | 0.879 ± 0.124 (n = 16) | 0.8292 ± 0.099 (n = 13) | 0.6257 |
| Phosphate | 1.498 ± 0.204 (n = 14) | 1.388 ± 0.253 (n = 12) | 0.2301 |
| Haemoglobin | 126.1 ± 12.15 (n = 15) | 121.3 ± 19.27 (n = 15) | 0.4151 |
| Vitamin D | 88.91 ± 25.33 (n = 13) | 85.45 ± 25.54 (n = 13) | 0.7534 |
| Vitamin B12 | 651.8 ± 333.0 (n = 6) | 559.2 ± 371.1 (n = 7) | 0.6476 |
| Nutrients | BTF (n = 12) | CF (n = 19) | p Value |
|---|---|---|---|
| kJ/kg | 325.4 ± 134.1 | 242.5 ± 261.1 | 0.016 * |
| Total KJ | 6436 ± 1711 | 5227 ± 1447 | 0.043 * |
| % kJ from Carbohydrates | 41.25 ± 7.208 | 49.90 ± 6.716 | 0.002 * |
| % kJ from Protein | 19.03 ± 5.004 | 10.75 ± 2.332 | <0.001 * |
| % kJ from Fat | 37.53 ± 4.775 | 38.99 ± 5.447 | 0.818 |
| Carbohydrate Total (g) | 157.4 ± 49.66 | 144.5 ± 59.20 | 0.536 |
| Protein (g) | 72.34 ± 24.51 | 32.62 ± 13.50 | <0.001 * |
| Fat (g) | 64.79 ± 20.00 | 53.23 ± 18.54 | 0.112 |
| Fibre (%RDI 1) | 119.4 ± 51.25 | 19.86 ± 30.38 | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekar, N.; Dehlsen, K.; Leach, S.T.; Krishnan, U. Blenderised Tube Feeds vs. Commercial Formula: Which Is Better for Gastrostomy-Fed Children? Nutrients 2022, 14, 3139. https://doi.org/10.3390/nu14153139
Chandrasekar N, Dehlsen K, Leach ST, Krishnan U. Blenderised Tube Feeds vs. Commercial Formula: Which Is Better for Gastrostomy-Fed Children? Nutrients. 2022; 14(15):3139. https://doi.org/10.3390/nu14153139
Chicago/Turabian StyleChandrasekar, Neha, Kate Dehlsen, Steven T. Leach, and Usha Krishnan. 2022. "Blenderised Tube Feeds vs. Commercial Formula: Which Is Better for Gastrostomy-Fed Children?" Nutrients 14, no. 15: 3139. https://doi.org/10.3390/nu14153139