Prevalence of Metabolic Syndrome and Association with Physical Activity and Frailty Status in Spanish Older Adults with Decreased Functional Capacity: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethics Statement
2.3. Diagnosis of Metabolic Syndrome
2.4. Biochemical Parameters
2.5. Blood Pressure
2.6. Body Composition and Anthropometric Measurements
2.7. Frailty Status
2.8. Physical Activity
2.9. Statistical Analysis
3. Results
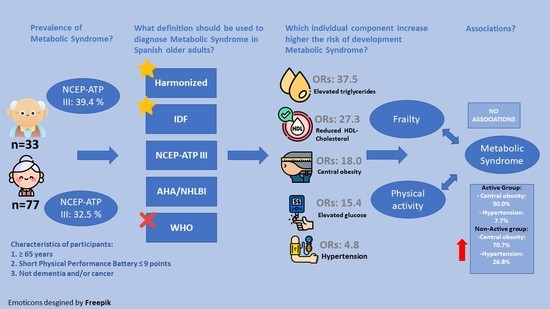
3.1. Prevalence of Metabolic Syndrome
3.2. Agreement and Disparity in Diagnosis of Metabolic Syndrome
3.3. Odds Ratios of Individual Components of Metabolic Syndrome
3.4. Association between Frailty Status and Metabolic Syndrome
3.5. Association between Physical Activity Behaviour and Metabolic Syndrome
4. Discussion
4.1. Prevalence of MetS
4.2. Prevalence of Individual Components of Metabolic Syndrome
4.3. Agreement and Disparity in Diagnosis of Metabolic Syndrome
4.4. Odds Ratios of Individual Components of Metabolic Syndrome
4.5. Association between Frailty Status and Metabolic Syndrome
4.6. Association between Physical Activity and Metabolic Syndrome
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 7 July 2021).
- Chen, M.Z.; Wong, M.W.K.; Lim, J.Y.; Merchant, R.A. Frailty and Quality of Life in Older Adults with Metabolic Syndrome—Findings from the Healthy Older People Everyday (HOPE) Study. J. Nutr. Health Aging 2021, 25, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Ng, O.C.; Wong, T.W.; Joseph, A.; Chan, Y.M.; Hejar, A.R. Prevalence of metabolic syndrome in type 2 diabetic patients: A comparative study using WHO, NCEP ATP III, IDF and Harmonized definitions. Health 2013, 5, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Serafino-Agrusa, L. Asthma and metabolic syndrome: Current knowledge and future perspectives. World J. Clin. Cases 2015, 3, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Uzunlulu, M.; Caklili, O.T.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and Metabolic Syndrome: A Tangled Web. Curr. Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome Pandemic. Arter. Thromb. Vasc. Biol. 2008, 28, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.W.; D’Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 158. [Google Scholar] [CrossRef] [Green Version]
- International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome; International Diabetes Federation: Brussels, Belgium, 2006. [Google Scholar]
- Golbidi, S.; Mesdaghinia, A.; Laher, I. Exercise in the Metabolic Syndrome. Oxid. Med. Cell. Longev. 2012, 2012, 349710. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 1999; pp. 31–33. Available online: https://apps.who.int/iris/handle/10665/66040 (accessed on 30 November 2021).
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The Metabolic Syndrome: A Global Public Health Problem and A New Definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirfaiz, S.; Shahril, M.R. Objectively Measured Physical Activity, Sedentary Behavior, and Metabolic Syndrome in Adults: Systematic Review of Observational Evidence. Metab. Syndr. Relat. Disord. 2019, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Report on Ageing and Health; World Health Organization: Geneva, Switzerland, 2015; ISBN 9789241565042. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [Green Version]
- Ekelund, U.; Griffin, S.J.; Wareham, N.J.; on behalf of the ProActive Research Group. Physical Activity and Metabolic Risk in Individuals with a Family History of Type 2 Diabetes. Diabetes Care 2007, 30, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Laaksonen, D.E.; Lakka, H.-M.; Salonen, J.T.; Niskanen, L.K.; Rauramaa, R.; Lakka, T.A. Low Levels of Leisure-Time Physical Activity and Cardiorespiratory Fitness Predict Development of the Metabolic Syndrome. Diabetes Care 2002, 25, 1612–1618. [Google Scholar] [CrossRef] [Green Version]
- Moreira, G.C.; Cipullo, J.P.; Ciorlia, L.A.S.; Cesarino, C.B.; Vilela-Martin, J.F. Prevalence of Metabolic Syndrome: Association with Risk Factors and Cardiovascular Complications in an Urban Population. PLoS ONE 2014, 9, e105056. [Google Scholar] [CrossRef]
- Higuita-Gutiérrez, L.F.; Quiroz, W.D.J.M.; Cardona-Arias, J.A. Prevalence of Metabolic Syndrome and Its Association with Sociodemographic Characteristics in Participants of a Public Chronic Disease Control Program in Medellin, Colombia, in 2018. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Ervin, R.B. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl. Health Stat. Rep. 2009, 5, 1–7. [Google Scholar]
- He, Y.; Jiang, B.; Wang, J.; Feng, K.; Chang, Q.; Fan, L.; Li, X.; Hu, F.B. Prevalence of the Metabolic Syndrome and its Relation to Cardiovascular Disease in an Elderly Chinese Population. J. Am. Coll. Cardiol. 2006, 47, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yan, H.; Yang, R.; Li, Q.; Dang, S.; Wang, Y. Prevalence and Determinants of Metabolic Syndrome among Adults in a Rural Area of Northwest China. PLoS ONE 2014, 9, e91578. [Google Scholar] [CrossRef]
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Reynolds, R.F.; Whelton, P.K.; He, J. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet 2005, 365, 1398–1405. [Google Scholar] [CrossRef]
- Fernández-García, I.; Gómez-Cabello, A.; Moradell, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; Ara, I.; Pedrero-Chamizo, R.; Subías-Perié, J.; Muniz-Pardos, B.; Casajús, J.A.; et al. How to Improve the Functional Capacity of Frail and Pre-Frail Elderly People? Health, Nutritional Status and Exercise Intervention. The EXERNET-Elder 3.0 Project. Sustainability 2020, 12, 6246. [Google Scholar] [CrossRef]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2017, 64, 61. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower Extremity Function and Subsequent Disability: Consistency Across Studies, Predictive Models, and Value of Gait Speed Alone Compared with the Short Physical Performance Battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cífková, R.; De Backer, G.; Dominiczak, A.; et al. Guía de práctica clínica de la ESH/ESC para el manejo de la hipertensión arterial. Hipertensión y Riesgo Vascular 2013, 30, 4–91. [Google Scholar] [CrossRef]
- Marfell-Jones, M.; Olds, T.; Stewart, A. International Standards for Anthropometric Assessment; ISAK: Potchefstroom, South Africa, 2011; ISBN 0620362073. [Google Scholar]
- Zhang, S.; Murray, P.; Zillmer, R.; Eston, R.G.; Catt, M.; Rowlands, A.V. Activity Classification Using the GENEA. Med. Sci. Sports Exerc. 2012, 44, 2228–2234. [Google Scholar] [CrossRef] [Green Version]
- Van Hees, V.T.; Renström, F.; Wright, A.; Gradmark, A.; Catt, M.; Chen, K.; Löf, M.; Bluck, L.; Pomeroy, J.; Wareham, N.J.; et al. Estimation of Daily Energy Expenditure in Pregnant and Non-Pregnant Women Using a Wrist-Worn Tri-Axial Accelerometer. PLoS ONE 2011, 6, e22922. [Google Scholar] [CrossRef] [Green Version]
- Sanders, G.J.; Boddy, L.M.; Sparks, A.; Curry, W.B.; Roe, B.; Kaehne, A.; Fairclough, S.J. Evaluation of wrist and hip sedentary behaviour and moderate-to-vigorous physical activity raw acceleration cutpoints in older adults. J. Sports Sci. 2018, 37, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Tasheva, P.; Kraege, V.; Vollenweider, P.; Roulet, G.; Méan, M.; Marques-Vidal, P. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness—An observational study. BMC Geriatr. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef]
- Barranco-Ruiz, Y.; Villa-González, E.; Venegas-Sanabria, L.C.; Chavarro-Carvajal, D.A.; Cano-Gutiérrez, C.A.; Izquierdo, M.; Correa-Bautista, J.E.; González-Ruíz, K.; Ramírez-Vélez, R. Metabolic Syndrome and Its Associated Factors in Older Adults: A Secondary Analysis of SABE Colombia in 2015. Metab. Syndr. Relat. Disord. 2020, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the Metabolic Syndrome Among US Adults. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Wang, X.; Howell, D.; Tang, L.; Shao, J.; Ye, Z. Comparative study on prevalence of metabolic syndrome based on three criteria among adults in Zhejiang province, China: An observational study. BMJ Open 2020, 10, e035216. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Mai, Z.; Zhou, S.; Liu, Y.; Li, S.; Zhao, Z.; Duan, X.; Cai, C.; Deng, T.; Zhu, W.; et al. Prevalence of metabolic syndrome in China: An up-dated cross-sectional study. PLoS ONE 2018, 13, e0196012. [Google Scholar] [CrossRef]
- Cai, R.; Chao, J.; Kong, L.; Wang, Y. Comparison of Four Different Metabolic Syndrome Diagnostic Criteria among the Elderly in Nanjing. Chin. J. Dis. Control Prev. 2019, 23, 146–149, 161. [Google Scholar]
- Guerreroromero, F.; Iacute, M.R.; Guezmoran, M.R. Concordance Between the 2005 International Diabetes Federation Definition for Diagnosing Metabolic Syndrome with the National Cholesterol Education Program Adult Treatment Panel III and the World Health Organization Definitions. Diabetes Care 2005, 28, 2588a–2589a. [Google Scholar] [CrossRef] [Green Version]
- Can, A.S.; Bersot, T.P. Analysis of agreement among definitions of metabolic syndrome in nondiabetic Turkish adults: A methodological study. BMC Public Health 2007, 7, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempegowda, P.; Marcus, S.R.; Solanki, P.; Reddy, R.S.; Nandini, D.R.; Dharmalingam, M. Prevalence of the metabolic syndrome in rural India—A disparity in definitions. Int. J. Diabetes Dev. Ctries 2011, 31, 188–193. [Google Scholar] [CrossRef]
- Wube, T.B.; Nuru, M.M.; Anbese, A.T. A Comparative Prevalence of Metabolic Syndrome Among Type 2 Diabetes Mellitus Patients in Hawassa University Comprehensive Specialized Hospital Using Four Different Diagnostic Criteria. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1877–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The Concept of Metabolic Syndrome: Contribution of Visceral Fat Accumulation and Its Molecular Mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Pérez-Aguilar, F. Etiopatogenia de la esteatohepatitis no alcohólica. Gastroenterología y Hepatología 2005, 28, 396–406. [Google Scholar] [CrossRef]
- Lewis, G.F.; Steiner, G. Acute Effects of Insulin in the Control of VLDL Production in Humans: Implications for the insulin-resistant state. Diabetes Care 1996, 19, 390–393. [Google Scholar] [CrossRef]
- Buchmann, N.; Spira, D.; König, M.; Demuth, I.; Steinhagen-Thiessen, E. Frailty and the Metabolic Syndrome—Results of the Berlin Aging Study II (BASE-II). J. Frailty Aging 2019, 8, 169–175. [Google Scholar] [CrossRef]
- Lang, I.A.; Llewellyn, D.J.; Alexander, K.; Melzer, D. Obesity, Physical Function, and Mortality in Older Adults. J. Am. Geriatr. Soc. 2008, 56, 1474–1478. [Google Scholar] [CrossRef]
- Buchmann, N.; Nikolov, J.; Spira, D.; Demuth, I.; Steinhagen-Thiessen, E.; Eckardt, R.; Norman, K. Identifying Sarcopenia in Metabolic Syndrome: Data from the Berlin Aging Study II. J. Gerontol. Ser. A 2015, 71, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Bailetti, D.; Sentinelli, F.; Prudente, S.; Cimini, F.A.; Barchetta, I.; Totaro, M.; Di Costanzo, A.; Barbonetti, A.; Leonetti, F.; Cavallo, M.G.; et al. Deep Resequencing of 9 Candidate Genes Identifies a Role for ARAP1 and IGF2BP2 in Modulating Insulin Secretion Adjusted for Insulin Resistance in Obese Southern Europeans. Int. J. Mol. Sci. 2022, 23, 1221. [Google Scholar] [CrossRef]
- Sehn, A.P.; Brand, C.; Silveira, J.F.D.C.; Andersen, L.B.; Gaya, A.R.; Todendi, P.F.; Valim, A.R.D.M.; Reuter, C.P. What is the role of cardiorespiratory fitness and sedentary behavior in relationship between the genetic predisposition to obesity and cardiometabolic risk score? BMC Cardiovasc. Disord. 2022, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sisson, S.B.; Camhi, S.M.; Church, T.S.; Tudor-Locke, C.; Johnson, W.; Katzmarzyk, P.T. Accelerometer-Determined Steps/Day and Metabolic Syndrome. Am. J. Prev. Med. 2010, 38, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cohen, S.A.; Lofgren, I.E.; Greene, G.W.; Delmonico, M.J.; Greaney, M.L. The Association between Physical Activity and Metabolic Syndrome in Older Adults with Obesity. J. Frailty Aging 2018, 8, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Parsons, T.J.; Sartini, C.; Ash, S.; Lennon, L.T.; Wannamethee, S.G.; Lee, I.-M.; Whincup, P.H. Does duration of physical activity bouts matter for adiposity and metabolic syndrome? A cross-sectional study of older British men. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Wewege, M.A.; Thom, J.; Rye, K.-A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef]
- Naci, H.; Ioannidis, J.P.A. Comparative effectiveness of exercise and drug interventions on mortality outcomes: Metaepidemiological study. BMJ 2013, 347, f5577. [Google Scholar] [CrossRef] [Green Version]


| WHO (1999) | NCEP-ATP III (2001) | AHA/NHLBI (2005) | IDF (2006) | Harmonized (2009) | |
|---|---|---|---|---|---|
| Definitions | Insulin resistance plus 2 additional risk factors | ≥3 of the following five risk factors: | ≥3 of the following five risk factors: | Central obesity plus 2 other features | ≥3 of the following five risk factors: |
| Elevated glucose | ≥6.1 mmol/L (110 mg/dL) or diagnosed T2DM | ≥6.1 mmol/L (110 mg/dL) or diagnosed T2DM | ≥5.6 mmol/L (100 mg/dL) or diagnosed T2DM | ≥5.6 mmol/L (100 mg/dL) or diagnosed T2DM | ≥5.6 mmol/L (100 mg/dL) or diagnosed T2DM |
| Central obesity | Men: WHR > 0.9 and/or BMI >30 kg/m2 | Men: WC > 102 cm | Men: WC > 102 cm | Men: WC > 94 cm or BMI > 30 kg/m2 | Ethnic cut point for WC (Mediterranean, WC > 94 cm) |
| Women: WHR > 0.85 and/or BMI >30 kg/m2 | Women: WC> 88 cm | Women: WC > 88 cm | Women: WC > 80 cm or BMI > 30 kg/m2 | Ethnic cut point for WC (Mediterranean, WC > 80 cm) | |
| Hypertension | ≥140/90 mmHg or treatment | ≥130/85 mmHg or treatment | ≥130/85 mmHg or treatment | ≥130/85 mmHg or treatment | ≥130/85 mmHg or treatment |
| Elevated TGs | ≥1.7 mmol/L (150 mg/dL) or treatment | ≥1.7 mmol/L (150 mg/dL) or treatment | ≥1.7 mmol/L (150 mg/dL) or treatment | ≥1.7 mmol/L (150 mg/dL) or treatment | ≥1.7 mmol/L (150 mg/dL) or treatment |
| Reduced HDL-C | Men: <0.9 mmol/L (35 mg/dL) or treatment | Men: <1.03 mmol/L (40 mg/dL) or treatment | Men: <1.03 mmol/L (40 mg/dL) or treatment | Men: <1.03 mmol/L 40 mg/dL) or treatment | Men: <1.03 mmol/L 40 mg/dL) or treatment |
| Women: <1.0 mmol/L (39 mg/dL) or treatment | Women: <1.29 mmol/L (50 mg/dL) or treatment | Women: <1.29 mmol/L (50 mg/dL) or treatment | Women: <1.29 mmol/L (50 mg/dL) or treatment | Women: <1.29 mmol/L (50 mg/dL) or treatment |
| Characteristic | Men (n = 33) n (%) or Mean ± SD | Women (n = 77) n (%) or Mean ± SD | p Value |
|---|---|---|---|
| Socio-demographic | |||
| Age (y.) | 80.5 ± 6.2 | 80.7 ± 5.7 | 0.838 |
| Fried- Frailty status | 0.015 | ||
| Robust | 12 (36.4%) | 11 (14.3%) | |
| Pre-frail | 17 (51.5%) | 49 (63.6%) | |
| Frail | 4 (12.1%) | 17 (22.1%) | |
| Physical activity * | 0.830 | ||
| Active | 15 (57.7%) | 30 (44.8%) | |
| Non-Active | 11 (42.3%) | 37 (55.2%) | |
| Anthropometrics and body composition | |||
| Weight (kg) | 81.6 ± 15.0 | 70.2 ± 13.4 | <0.001 |
| Height (cm) | 166.7 ± 6.7 | 153.8 ± 6.2 | <0.001 |
| BMI (kg/m2) | 29.2 ± 5.5 | 30.0 ± 5.7 | 0.581 |
| Body fat (%) | 30.8 ± 6.1 | 39.5 ± 6.3 | <0.001 |
| WC (cm) | 102.5 ± 12.7 | 92.7 ± 12.2 | 0.001 |
| Hip circumference (cm) | 103.8 ± 9.4 | 105.1 ± 10.4 | 0.639 |
| WHR | 0.96 ± 0.05 | 0.86 ± 0.07 | <0.001 |
| Serum Biochemical | |||
| Glucose (mg/dL) | 103.6 ± 15.6 | 105.8 ± 29.6 | 0.696 |
| HDL-C (mg/dL) | 48.8 ± 17.2 | 55.1 ± 15.9 | 0.080 |
| TGs (mg/dL) | 124.0 ± 64.4 | 131.1 ± 53.6 | 0.567 |
| Blood pressure | |||
| SBP (mmHg) | 136.0 ± 20.7 | 136.9 ± 15.6 | 0.803 |
| DBP (mmHg) | 73.9 ± 12.7 | 73.5 ± 9.4 | 0.858 |
| Components of MetS | WHO | NCEP-ATP III | AHA/NHLBI | IDF | Harmonized | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | p Value | Men | Women | p Value | Men | Women | p Value | Men | Women | p Value | Men | Women | p Value | |
| Elevated glucose | 10 (30.3%) | 23 (29.9%) | 0.929 | 10 (30.3%) | 23 (29.9%) | 0.929 | 16 (48.5%) | 31 (40.3%) | 0.275 | 16 (48.5%) | 31 (40.3%) | 0.275 | 16 (48.5%) | 31 (40.3%) | 0.275 |
| Central obesity * | 16 (88.9%) | 32 (57.1%) | <0.001 | 16 (48.5%) | 49 (63.6%) | 0.115 | 16 (48.5%) | 49 (63.6%) | 0.115 | 21 (63.6%) | 66 (85.7%) | <0.001 | 21 (63.6%) | 66 (85.7%) | <0.001 |
| Hypertension ** | 6 (18.2%) | 9 (11.7%) | 0.081 | 6 (18.2%) | 9 (11.7%) | 0.081 | 6 (18.2%) | 9 (11.7%) | 0.081 | 6 (18.2%) | 9 (11.7%) | 0.081 | 6 (18.2%) | 9 (11.7%) | 0.081 |
| Elevated TGs | 8 (24.2%) | 25 (32.5%) | 0.067 | 8 (24.2%) | 25 (32.5%) | 0.067 | 8 (24.2%) | 25 (32.5%) | 0.067 | 8 (24.2%) | 25 (32.5%) | 0.067 | 8 (24.2%) | 25 (32.5%) | 0.067 |
| Reduced HDL-C | 7 (21.2%) | 11 (14.3%) | 0.086 | 11 (33.3%) | 29 (37.7%) | 0.314 | 11 (33.3%) | 29 (37.7%) | 0.314 | 11 (33.3%) | 29 (37.7%) | 0.314 | 11 (33.3%) | 29 (37.7%) | 0.314 |
| Total prevalence of MetS by sex | 4 (22.2%) | 10 (17.5%) | 0.483 | 13 (39.4%) | 25 (32.5%) | 0.214 | 10 (30.3%) | 28 (36.4%) | 0.196 | 11 (33.3%) | 30 (39%) | 0.236 | 11 (33.3%) | 30 (39%) | 0.236 |
| Total prevalence of MetS | 14 (15.7%) | 38 (34.5%) | 38 (34.5%) | 41 (37.3%) | 41 (37.3%) | ||||||||||
| Criteria | NCEP-ATP III | Sensitivity (%) | Specificity (%) | Kappa Index | Agreement | ||
|---|---|---|---|---|---|---|---|
| MetS | Non-MetS | ||||||
| WHO (n = 89) | MetS | 12 | 2 | 0.387 | 0.965 | 0.404 | Fair |
| Non-MetS | 19 | 56 | |||||
| AHA/NHLBI (n = 110) | MetS | 34 | 4 | 1 | 0.947 | 0.918 | Very good |
| Non-MetS | 0 | 72 | |||||
| IDF * (n = 110) | MetS | 34 | 7 | 1 | 0.908 | 0.859 | Good |
| Non-MetS | 0 | 69 | |||||
| Harmonized * (n = 110) | MetS | 34 | 7 | 1 | 0.908 | 0.859 | Good |
| Non-MetS | 0 | 69 | |||||
| Individual Components of MetS (Prevalence in the Whole Sample) | Yes/No | Prevalence of MetS within Each Group (%) | Odds Ratio (95% CI) | Kappa Index |
|---|---|---|---|---|
| Elevated glucose (30%) | Yes | 75.8 | 15.4 (5.7–41.6) | 0.564 |
| No | 16.9 | |||
| Central obesity (65.5%) | Yes | 50.0 | 18.0 (4.0–80.4) | 0.369 |
| No | 5.3 | |||
| Hypertension (13.6%) | Yes | 66.7 | 4.8 (1.5–15.3) | 0.226 |
| No | 29.5 | |||
| Elevated TGs (33%) | Yes | 84.8 | 37.5 (11.7–119.8) | 0.689 |
| No | 13.0 | |||
| Reduced HDL-C (42.7%) | Yes | 86.8 | 27.3 (9.0–82.7) | 0.638 |
| No | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subías-Perié, J.; Navarrete-Villanueva, D.; Fernández-García, Á.I.; Moradell, A.; Gesteiro, E.; Pérez-Gómez, J.; Ara, I.; Vicente-Rodríguez, G.; Casajús, J.A.; Gómez-Cabello, A. Prevalence of Metabolic Syndrome and Association with Physical Activity and Frailty Status in Spanish Older Adults with Decreased Functional Capacity: A Cross-Sectional Study. Nutrients 2022, 14, 2302. https://doi.org/10.3390/nu14112302
Subías-Perié J, Navarrete-Villanueva D, Fernández-García ÁI, Moradell A, Gesteiro E, Pérez-Gómez J, Ara I, Vicente-Rodríguez G, Casajús JA, Gómez-Cabello A. Prevalence of Metabolic Syndrome and Association with Physical Activity and Frailty Status in Spanish Older Adults with Decreased Functional Capacity: A Cross-Sectional Study. Nutrients. 2022; 14(11):2302. https://doi.org/10.3390/nu14112302
Chicago/Turabian StyleSubías-Perié, Jorge, David Navarrete-Villanueva, Ángel Iván Fernández-García, Ana Moradell, Eva Gesteiro, Jorge Pérez-Gómez, Ignacio Ara, Germán Vicente-Rodríguez, José Antonio Casajús, and Alba Gómez-Cabello. 2022. "Prevalence of Metabolic Syndrome and Association with Physical Activity and Frailty Status in Spanish Older Adults with Decreased Functional Capacity: A Cross-Sectional Study" Nutrients 14, no. 11: 2302. https://doi.org/10.3390/nu14112302