Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review
Abstract
:1. Introduction
2. Materials and Methods
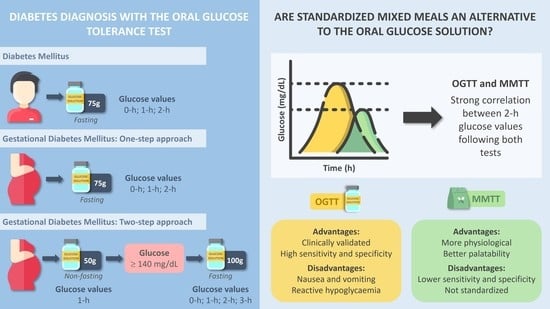
3. Standard Methods for the Diagnosis of Diabetes
4. Oral Glucose Tolerance Test and Mixed Meal Tolerance Tests
5. Mixed Meal Tolerance Test as a Method to Screen Glucose Disturbances
6. Meal Tolerance Tests to Screen for Gestational Diabetes Mellitus
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; pp. 36–43. [Google Scholar]
- Raposo, J.F. Diabetes: Factos e Números 2016, 2017 e 2018. Rev. Port. Diabetes 2020, 15, 19–27. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis epidemiology, pathophysiology, and management. J. Am. Med. Assoc. 2002, 287, 2570–2581. [Google Scholar] [CrossRef]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; Available online: http://apps.who.int/iris/bitstream/handle/10665/43588/9241594934_eng.pdf?sequence=1 (accessed on 4 February 2022).
- Daniells, S.; Grenyer, B.F.S.; Davis, W.S.; Coleman, K.J.; Burgess, J.A.P.; Moses, R.G. Gestational diabetes mellitus: Is a diagnosis associated with an increase in maternal anxiety and stress in the short and intermediate term? Diabetes Care 2003, 26, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Hillier, T.A.; Vesco, K.K.; Pedula, K.L.; Beil, T.L.; Whitlock, E.P.; Pettitt, D.J. Screening for gestational diabetes mellitus: A systematic review for the U.S. preventive services task force. Ann. Intern. Med. 2008, 148, 766–775. [Google Scholar] [CrossRef]
- Brodovicz, K.G.; Girman, C.J.; Simonis-Bik, A.M.C.; Rijkelijkhuizen, J.M.; Zelis, M.; Bunck, M.C.; Mari, A.; Nijpels, G.; Eekhoff, E.M.W.; Dekker, J.M. Postprandial metabolic responses to mixed versus liquid meal tests in healthy men and men with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 94, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, F.Q.; Gannon, M.C.; Wald, J.L.; Ahmed, M. Plasma glucose and insulin profiles in normal subjects ingesting diets of varying carbohydrate, fat, and protein content. J. Am. Coll. Nutr. 1982, 4, 437–450. [Google Scholar] [CrossRef]
- Nuttall, F.Q.; Mooradian, A.D.; Gannon, M.C.; Billington, C.J.; Krezowski, P. Effect of protein ingestion on the glucose and insulin response to a standardized oral glucose load. Diabetes Care 1984, 7, 465–470. [Google Scholar] [CrossRef]
- Denis McGarry, J. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [PubMed] [Green Version]
- Meier, J.J.; Baller, B.; Menge, B.A.; Gallwitz, B.; Schmidt, W.E.; Nauck, M.A. Excess glycaemic excursions after an oral glucose tolerance test compared with a mixed meal challenge and self-measured home glucose profiles: Is the OGTT a valid predictor of postprandial hyperglycaemia and vice versa? Diabetes Obes. Metab. 2009, 11, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.R.; Poppitt, S.D. HbA1c as a Marker of Prediabetes: A Reliable Screening Tool or Not. Insights Nutr. Metab. 2017, 1, 11–20. [Google Scholar]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and Effectiveness of Screen and Treat Policies in Prevention of Type 2 Diabetes: Systematic Review and Meta-Analysis of Screening Tests and Interventions. BMJ 2017, 356, i6538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorcely, B.; Katz, K.; Jagannathan, R.; Chiang, S.S.; Oluwadare, B.; Goldberg, I.J.; Bergman, M. Novel Biomarkers for Prediabetes, Diabetes, and Associated Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 345–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hostalek, U. Global Epidemiology of Prediabetes—Present and Future Perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.K.O.; Xue, H.; Pang, E.W.H.; Tam, D.C.C. Accuracy of fasting plasma glucose and hemoglobin A1c testing for the early detection of diabetes: A pilot study. Front. Lab. Med. 2017, 1, 76–81. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient Regulation of Insulin Secretion and Action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef]
- Bolli, G.B.; de Feo, P.; de Cosmo, S.; Perriello, G.; Ventura, M.M.; Calcinaro, F.; Lolli, C.; Campbell, P.; Brunetti, P.; Gerich, J.E. Demonstration of a Dawn Phenomenon in Normal Human Volunteers. Diabetes 1984, 33, 1150–1153. [Google Scholar] [CrossRef]
- Troisi, R.J.; Cowie, C.C.; Harris, M.I. Diurnal Variation in Fasting Plasma Glucose: Implications for Diagnosis of Diabetes in Patients Examined in the Afternoon. JAMA 2000, 284, 3157–3159. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Ko, S.H.; Kwon, H.S.; Kim, N.H.; Kim, J.H.; Kim, C.S.; Song, K.H.; Won, J.C.; Lim, S.; Choi, S.H.; et al. Prevalence of Diabetes and Prediabetes according to Fasting Plasma Glucose and HbA1c. Diabetes Metab. J. 2013, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.T.; Howard, B.V.; Go, O.; Savage, P.J.; Fabsitz, R.R.; Robbins, D.C.; Welty, T.K. Prevalence of Undiagnosed Diabetes in Three American Indian Populations. A Comparison of the 1997 American Diabetes Association Diagnostic Criteria and the 1985 World Health Organization Diagnostic Criteria: The Strong Heart Study. Diabetes Care 2000, 23, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bahrani, A.I.; Charles, B.; Raid, B.; Al-Yahyaee, S.A. Diagnostic Accuracy of the American Diabetes Association Criteria in the Diagnosis of Glucose Intolerance among High-Risk Omani Subjects. Ann. Saudi Med. 2004, 24, 183–185. [Google Scholar]
- American Diabetes Association Professionals Committee Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [CrossRef] [PubMed]
- Gonzalez, A.; Deng, Y.; Lane, A.N.; Benkeser, D.; Cui, X.; Staimez, L.R.; Ford, C.N.; Khan, F.N.; Markley Webster, S.C.; Leong, A.; et al. Impact of Mismatches in HbA1c vs Glucose Values on the Diagnostic Classification of Diabetes and Prediabetes. Diabet. Med. 2020, 37, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2012, 9, 25–53. [Google Scholar] [CrossRef]
- Nolan, C.J.; Prentki, M. The Islet β-Cell: Fuel Responsive and Vulnerable. Trends Endocrinol. Metab. 2008, 19, 285–291. [Google Scholar] [CrossRef]
- Newsholme, P.; Krause, M. Nutritional Regulation of Insulin Secretion: Implications for Diabetes. Clin. Biochem. Rev. 2012, 33, 35. [Google Scholar]
- Desouza, C.; Salazar, H.; Cheong, B.; Murgo, J.; Fonseca, V. Association of Hypoglycemia and Cardiac Ischemia. Diabetes Care 2003, 26, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Miura, J.; Uchigata, Y.; Sato, A.; Matsunaga, R.; Fujito, T.; Borgeld, H.-J.; Tanaka, M.; Babazono, T.; Takahashi, C.; Iwamoto, Y. An IDDM Patient Who Complained of Chest Oppression with Ischemic Changes on ECG in Insulin-Induced Hypoglycemia. Diabetes Res. Clin. Pract. 1998, 39, 31–37. [Google Scholar] [CrossRef]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International Consensus on the Diagnosis and Management of Dumping Syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.W.; Saslow, S.B.; Camilleri, M.; Thomforde, G.M.; Dinneen, S.; Rizza, R.A. Mechanism of Accelerated Gastric Emptying of Liquids and Hyperglycemia in Patients with Type II Diabetes Mellitus. Gastroenterology 1995, 109, 755–765. [Google Scholar] [CrossRef]
- Nesti, L.; Mengozzi, A.; Tricò, D. Impact of Nutrient Type and Sequence on Glucose Tolerance: Physiological Insights and Therapeutic Implications. Front. Endocrinol. 2019, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Brooks/Cole Publishing: Pacific Groove, CA, USA, 1996. [Google Scholar]
- Chanprasertpinyo, W.; Bhirommuang, N.; Surawattanawiset, T.; Tangsermwong, T.; Phanachet, P.; Sriphrapradang, C. Using Ice Cream for Diagnosis of Diabetes Mellitus and Impaired Glucose Tolerance: An Alternative to the Oral Glucose Tolerance Test. Am. J. Med. Sci. 2017, 354, 581–585. [Google Scholar] [CrossRef]
- Wolever, T.M.S.; Chiasson, J.L.; Csima, A.; Hunt, J.A.; Palmason, C.; Ross, S.A.; Ryan, E.A. Variation of Postprandial Plasma Glucose, Palatability, and Symptoms Associated with a Standardized Mixed Test Meal versus 75 g Oral Glucose. Diabetes Care 1998, 21, 336–340. [Google Scholar] [CrossRef]
- Marena, S.; Montegrosso, G.; de Michieli, F.; Pisu, E.; Pagano, G. Comparison of the Metabolic Effects of Mixed Meal and Standard Oral Glucose Tolerance Test on Glucose, Insulin and C-Peptide Response in Healthy, Impaired Glucose Tolerance, Mild and Severe Non-Insulin-Dependent Diabetic Subjects. Acta Diabetol. 1992, 29, 29–33. [Google Scholar] [CrossRef]
- Harano, Y.; Miyawaki, T.; Nabiki, J.; Shibachi, M.; Adachi, T.; Ikeda, M.; Ueda, F.; Nakano, T. Development of Cookie Test for the Simultaneous Determination of Glucose Intolerance, Hyperinsulinemia, Insulin Resistance and Postprandial Dyslipidemia. Endocr. J. 2006, 53, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Traub, M.L.; Jain, A.; Maslow, B.-S.; Pal, L.; Stein, D.T.; Santoro, N.; Freeman, R. The “Muffin Test”—An Alternative to the Oral Glucose Tolerance Test for Detecting Impaired Glucose Tolerance. Menopause 2012, 19, 62–66. [Google Scholar] [CrossRef]
- Freeman, R.; Pollack, R.; Rosenbloom, E. Assessing Impaired Glucose Tolerance and Insulin Resistance in Polycystic Ovarian Syndrome with A Muffin Test: An Alternative to the Glucose Tolerance Test. Endocr. Pract. 2010, 16, 810–817. [Google Scholar] [CrossRef]
- Forbes, S.; Lam, A.; Koh, A.; Imes, S.; Dinyari, P.; Malcolm, A.J.; Shapiro, A.M.J.; Senior, P.A. Comparison of Metabolic Responses to the Mixed Meal Tolerance Test vs the Oral Glucose Tolerance Test after Successful Clinical Islet Transplantation. Clin. Transplant. 2018, 32, e13301. [Google Scholar] [CrossRef]
- Marais, C.; Hall, D.R.; van Wyk, L.; Conradie, M. Randomized Cross-Over Trial Comparing the Diagnosis of Gestational Diabetes by Oral Glucose Tolerance Test and a Designed Breakfast Glucose Profile. Int. J. Gynecol. Obstet. 2018, 141, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Racusin, D.A.; Antony, K.; Showalter, L.; Sharma, S.; Haymond, M.; Aagaard, K.M. Candy Twists as an Alternative to the Glucola Beverage in Gestational Diabetes Mellitus Screening. Am. J. Obstet. Gynecol. 2015, 212, 522.e1–522.e5. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.N.; McManus, J.; Dobbs, S.; Hadden, D.R. A Standardised Breakfast Tolerance Test in Pregnancy: Comparison with the 75 g Oral Glucose Tolerance Test in Unselected Mothers and in Those with Impaired Glucose Tolerance. Ulster Med. J. 1997, 66, 18. [Google Scholar] [PubMed]
- Coustan, D.R.; Widness, J.A.; Carpenter, M.W.; Rotondo, L.; Pratt, D.C. The “Breakfast Tolerance Test”: Screening for Gestational Diabetes with a Standardized Mixed Nutrient Meal. Am. J. Obstet. Gynecol. 1987, 157, 1113–1117. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2008, 31, S55–S60. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, M.W.; Coustan, D.R. Criteria for Screening Tests for Gestational Diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773. [Google Scholar] [CrossRef]
- Eslamian, L.; Ramezani, Z. Breakfast as a Screening Test for Gestational Diabetes. Int. J. Gynecol. Obstet. 2006, 96, 34–35. [Google Scholar] [CrossRef]
- Group, N.D.D. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar]
- World Health Organization. WHO Expert Committee on Diabetes Mellitus: Second Report; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1980; Volume 646, Available online: https://apps.who.int/iris/handle/10665/41399 (accessed on 10 January 2022).
- Lind, T.; Phillips, P.R. Diabetic Pregnancy Study Group of the European Association for the Study of Diabetes Influence of Pregnancy on the 75-g OGTT: A Prospective Multicenter Study. Diabetes 1991, 40, 8–13. [Google Scholar] [CrossRef]
- Marais, C.; van Wyk, L.; Conradie, M.; Hall, D. Screening for Gestational Diabetes: Examining a Breakfast Meal Test. S. Afr. J. Clin. Nutr. 2016, 29, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.J.; Kemmeries, G.; Holst, J.J.; Nauck, M.A. Erythromycin Antagonizes the Deceleration of Gastric emptying by Glucagon-Like Peptide 1 and Unmasks Its Insulinotropic Effect in Heathy Subjects. Diabetes 2005, 54, 2212–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, J.J.; Gallwitz, B.; Salmen, S.; Goetze, O.; Holst, J.J.; Schmidt, W.E.; Nauck, M.A. Normalization of Glucose Concentrations and Deceleration of Gastric Emptying after Solid Meals during Intravenous Glucagon-Like Peptide 1 in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2003, 88, 2719–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horowitz, M.; Harding, P.E.; Maddox, A.F.; Wishart, J.M.; Akkermans, L.M.A.; Chatterton, B.E.; Shearman, D.J.C. Gastric and Oesophageal Emptying in Patients with Type 2 (Non-Insulin-Dependent) Diabetes Mellitus. Diabetologia 1989, 32, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijkelijkhuizen, J.M.; McQuarrie, K.; Girman, C.J.; Stein, P.P.; Mari, A.; Holst, J.J.; Nijpels, G.; Dekker, J.M. Effects of Meal Size and Composition on Incretin, α-Cell, and β-Cell Responses. Metabolism. 2010, 59, 502–511. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Martens, M.J.I.; Lemmens, S.G.T.; Born, J.M.; Westerterp-Plantenga, M.S. Satiating Capacity and Post-Prandial Relationships between Appetite Parameters and Gut-Peptide Concentrations with Solid and Liquefied Carbohydrate. PLoS ONE 2012, 7, e42110. [Google Scholar] [CrossRef] [Green Version]
- Lachmann, E.H.; Fox, R.A.; Dennison, R.A.; Usher-Smith, J.A.; Meek, C.L.; Aiken, C.E. Barriers to Completing Oral Glucose Tolerance Testing in Women at Risk of Gestational Diabetes. Diabet. Med. 2020, 37, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Frid, A.H.; Nilsson, M.; Holst, J.J.; Björck, I.M. Effect of Whey on Blood Glucose and Insulin Responses to Composite Breakfast and Lunch Meals in Type 2 Diabetic Subjects 1–3. 2005. Available online: https://academic.oup.com/ajcn/article-abstract/82/1/69/4863431 (accessed on 2 March 2022).
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahrén, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef]
- Vilsbøll, T.; Krarup, T.; Sonne, J.; Madsbad, S.; Vølund, A.; Juul, A.G.; Holst, J.J. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2003, 88, 2706–2713. [Google Scholar] [CrossRef] [Green Version]
- Alssema, M.; Schindhelm, R.K.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Teerlink, T.; Nijpels, G.; Heine, R.J.; Dekker, J.M. Meal composition affects insulin secretion in women with type 2 diabetes: A comparison with healthy controls. The Hoorn prandial study. Eur. J. Clin. Nutr. 2009, 63, 398–404. [Google Scholar] [CrossRef]
- Heinemann, L. Are All Glucose Solutions Used for oGTT Equal? Diabet. Med. 2022, 39, e14798. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Participants | Main Results | 2-h Glucose Correlations |
|---|---|---|---|
| Randomized cross-over studies | |||
| Chanprasertpinyo et al. 2017 [37] | Healthy adults without DM (n = 104) 30 M; 74 F | 2-h glucose levels (OGTT/ice cream * test): ρ = 0.82; p < 0.001; 9.61% discordant diagnostic results Ice cream test: 5.76% of missed DM cases | + Very strong |
| Wolever et al. 1998 [38] | Adults with normal weight, obesity, IGT, or diabetes (n = 36) 15 M; 21 F | 2-h glucose levels (OGTT/MMTT): r = 0.97) 1-h glucose (MMTT)/2-h glucose (OGTT): r = 0.96 1-h glucose (OGTT)/2-h glucose (MMTT): r = 0.91 | + Very strong |
| Marena et al. 1992 [39] | Adults with NGT, IGT, mild NIDDM, or NIDDM (n = 40; 10 by group) 20 M; 20 F | Glucose incremental areas (OGTT/mixed meal *): r = 0.511, p < 0.001 2-h glucose values (OGTT/mixed meal): r = 0.956, p < 0.001 | + Very strong |
| Non-randomized cross-over studies | |||
| Meier et al. 2009 [14] | Adults with NGT, IGT, or diabetes (n = 60) | 2-h glucose levels (OGTT/MMTT): r2 = 0.78, p < 0.0001 | + Strong |
| Harano et al. 2006 [40] | Healthy adults (n = 19) 6 M; 13 F | Cookie * test: 1 (5%) IGT OGTT: 19 (100%) normal blood glucose | n.s. |
| Cross-sectional studies | |||
| Traub et al. 2012 [41] | Healthy early postmenopausal women (n = 12) | MMTT: 1 (8%) of 2 (16%) participants identified with IGT (confirmed with the OGTT). The second participant had abnormal fasting glucose with the OGTT | n.s. |
| Freeman et al. 2010 [42] | Women with PCOS (n = 8) | Blood glucose levels OGTT and MMTT: 1 (12%) IGT OGTT: 1 (12%) IFG (not with MMTT) MMTT: 1 (12%) diabetes (not with OGTT) Blood insulin levels OGTT and MMTT: 4 (57%) IGT | n.s. |
| Retrospective study | |||
| Forbes et al. 2018 [43] | Adults with T1DM and stable transplant grafts (n = 13) 9 M; 4 F | 2-h glucose values (OGTT and MMTT *): r = 0.45; p = 0.07 90-min MMTT glucose ≥ 144 mg/dL: equivalent to 2-h OGTT glucose ≥ 199.8 mg/dL | + Moderate |
| Ref. | Product | Energy (kcal) | Carbohydrates (% TE, g) | Protein (% TE, g) | Fat (% TE, g) |
|---|---|---|---|---|---|
| Forbes et al., 2018 [43] | Ensure HP | 1.1 kcal/mL | 55% | 22% | 23% |
| Marais et al., 2018 [44] | Future Life Excel meal | n.d. | 75.0 g | n.d. | n.d. |
| Chanprasertpinyo et al., 2017 [37] | Ice cream | 620.9 | 73.9 g | 18.9 g | 27.7 g |
| Racusin et al., 2015 [45] | 10 strawberry-flavored candy twists (Twizzlers) | n.d. | 50.0 g (91.968%) | 3.515% | 4.527% |
| Traub et al., 2012 [41] | Muffin (Beigel’s Bakery) | 410.0 | 56.0 g | 6.0 g | 18.0 g |
| Traub et al., 2012 [41] | Shake | 600.0 | 75.0 g | 30.0 g | 20.0 g |
| Freeman et al., 2010 [42] | Muffin (Costco) and orange juice (Tropicana) | 800.0 | 105.0 g | 12.0 g | 38.0 g |
| Meier et al., 2009 [14] | Continental breakfast 1 | 820.0 | 90.0 g | 26.8 g | 39.2 g |
| Harano et al., 2006 [40] | Cookie | 533.0 | 75.0 g | 7.0 g | 25.0 g |
| Wolever et al., 1998 [38] | 5 wafers (DSP) | 345.0 | 50.0 g | 12.1 g | 10.7 g |
| Roberts et al., 1997 [46] | Standardized breakfast 2 | 300.0 | 45.0 g | 10.0 g | 9.0 g |
| Marena et al., 1992 [39] | Standard mixed meal 3 | 590.0 | 69.0 g (44.0%) | 22.6 g (15.0%) | 27.0 g (41.0%) |
| Coustan et al., 1987 [47] | Standard test breakfast 4 | 600.0 | 52.0 g | 28.0 g | 31.0 g |
| Ref. | Participants | Main Results | 2-h Glucose Correlations |
|---|---|---|---|
| Case-control study | |||
| Eslamian and Ramezani 2006 [50] | Pregnant women (n = 141) | GCT: 41 (29.3%) GDM OGTT: 12 (8.57%) GDM Breakfast test: 28 (20%) GDM Optimal cut-off value: 130 mg/dL at 60 min (83.3% sensitivity; 85.9% specificity; 35.7% PPV; 98.2% NPV) | n.s. |
| Randomized cross-over studies | |||
| Marais et al., 2018 [44] | Pregnant women with a high risk of GDM (n = 51) | 2-h OGTT (venous): 5 (10%) GDM 2-h OGTT (capillary): 6 (12%) GDM 2-h DBGP test (capillary): 7 (14%) GDM; 3 (6%) missed GDM cases; 5 (10%) false-positive cases DBGP test: 25% sensitivity; 96% specificity; 33% PPV; 95% NPV | n.s. |
| Coustan et al., 1987 [47] | Pregnant women with GDM (n = 20) | 16 (80%) of the 20 subjects with GDM had a 1-h breakfast test plasma glucose level ≥ 120 mg/dL (threshold defined by the 1-h mean glucose + 2 SD) MMTT: 75% sensitivity; 94% specificity | n.s. |
| Non-randomized cross-over studies | |||
| Racusin et al., 2015 [45] | Pregnant women screened positive for GDM (n = 20) | 1-h candy twists test: 100% sensitivity; 50% specificity; 18% PPV; 100% NPV; 82% false-referral rate; 18% detection rate | + Moderate ** |
| Roberts et al., 1997 [46] | Non-diabetic pregnant women (n = 102) | OGTT (cut-off 144 mg/dL): 7 (7%) IGT Breakfast (cut-off 144 mg/dL): 0 (0%) IGT OGTT (cut-off 162 mg/dL): 2 (2%) IGT OGTT (cut off 192 mg/dL): 0 (0%) GDM | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lages, M.; Barros, R.; Moreira, P.; Guarino, M.P. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients 2022, 14, 2032. https://doi.org/10.3390/nu14102032
Lages M, Barros R, Moreira P, Guarino MP. Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review. Nutrients. 2022; 14(10):2032. https://doi.org/10.3390/nu14102032
Chicago/Turabian StyleLages, Marlene, Renata Barros, Pedro Moreira, and Maria P. Guarino. 2022. "Metabolic Effects of an Oral Glucose Tolerance Test Compared to the Mixed Meal Tolerance Tests: A Narrative Review" Nutrients 14, no. 10: 2032. https://doi.org/10.3390/nu14102032