How Are Infants Suspected to Have Cow’s Milk Allergy Managed? A Real World Study Report
Abstract
:1. Introduction
2. Materials and Methods
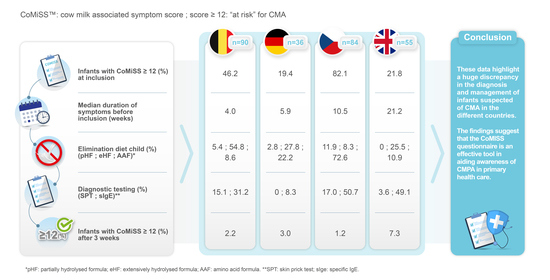
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandenplas, Y.; Al-Hussaini, B.; Al-Mannaei, K.; Al-Sunaid, A.; Helmi Ayesh, W.; El-Degeir, M.; El-Kabbany, N.; Haddad, J.; Hashmi, A.; Kreishan, F.; et al. Prevention of Allergic Sensitization and Treatment of Cow’s Milk Protein Allergy in Early Life: The Middle-East Step-Down Consensus. Nutrients 2019, 11, 1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R.; Chebar Lozinsky, A.; Fleischer, D.M.; Vieira, M.C.; Du Toit, G.; Vandenplas, Y.; Dupont, C.; Knibb, R.; Uysal, P.; Cavkaytar, O.; et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants-An EAACI Position Paper. Allergy 2020, 75, 14–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A workshop report on the development of the Cow’s Milk-related Symptom Score awareness tool for young children. Acta Paediatr. 2015, 104, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.; Groetch, M.; Venter, C. When Should Infants with Cow’s Milk Protein Allergy Use an Amino Acid Formula? A Practical Guide. J. Allergy Clin. Immunol. Pract. 2018, 6, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, S.; Niggemann, B. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Steutel, N.F.; Zeevenhooven, J.; Scarpato, E.; Vandenplas, Y.; Tabbers, M.M.; Staiano, A.; Benninga, M.A. Prevalence of Functional Gastrointestinal Disorders in European Infants and Toddlers. J. Pediatr. 2020, 221, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bellaiche, M.; Ategbo, S.; Krumholz, F.; Ludwig, T.; Miqdady, M.; Abkari, A.; Vandenplas, Y. A large-scale study to describe the prevalence, characteristics and management of functional gastrointestinal disorders in African infants. Acta Paediatr. 2020, 109, 2366–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease-Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Koletzko, S.; Isolauri, E.; Hill, D.; Oranje, A.P.; Brueton, M.; Staiano, A.; Dupont, C. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch. Dis. Child. 2007, 92, 902–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, R. Nutritional disorders resulting from food allergy in children. Pediatr. Allergy Immunol. 2018, 29, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, C.; Szajewska, H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur. J. Pediatr. 2015, 174, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; Abuabat, A.; Al-Hammadi, S.; Aly, G.S.; Miqdady, M.S.; Shaaban, S.Y.; Torbey, P.H. Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow’s Milk Protein Allergy. Pediatr. Gastroenterol. Hepatol. Nutr. 2014, 17, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D.; Althera Study Group. Treating cow’s milk protein allergy: A double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carvajal, E.; Peeters, S.; Balduck, N.; Jaddioui, Y.; Ribes-Koninckx, C.; Huysentruyt, K. The Cow’s Milk-Related Symptom Score (CoMiSSTM): Health Care Professional and Parent and Day-to-Day Variability. Nutrients 2020, 12, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigorajska, K.; Filipiak, Z.; Winiarska, P.; Adamiec, A.; Trent, B.; Vandenplas, Y.; Ruszczyński, M.; Szajewska, H. Cow’s Milk-Related Symptom Score in Presumed Healthy Polish Infants Aged 0-6 Months. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Bertoni, E.; Bogni, F.; Bonaita, V.; Armano, C.; Moretti, A.; Baù, M.; Luini, C.; D’Auria, E.; Marinoni, M.; et al. Testing the Cow’s Milk-Related Symptom Score (CoMiSSTM) for the Response to a Cow’s Milk-Free Diet in Infants: A Prospective Study. Nutrients 2019, 11, 2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Symptom | Score | |||
|---|---|---|---|---|
| Crying | 0 | ≤1 h/day | ||
| 1 | 1 to 1.5 h/day | |||
| 2 | 1.5 to 2 h/day | |||
| 3 | 2 to 3 h/day | |||
| 4 | 3 to 4 h/day | |||
| 5 | 4 to 5 h/day | |||
| 6 | ≥5 h/day | |||
| Regurgitation | 0 | 0 to 2 episodes/day | ||
| 1 | ≥3 to ≤5 episodes of small volume | |||
| 2 | >5 episodes of >1 coffee spoon | |||
| 3 | >5 episodes of ±half of the feedings in <half of the feedings | |||
| 4 | continuous regurgitations of small volumes >30 min after each feeding | |||
| 5 | regurgitation of half to complete volume of a feeding in at least half of the feedings | |||
| 6 | regurgitation of the complete volume after each feeding | |||
| Stools (Bristol scale) | 4 | type 1 and 2 (hard stools) | ||
| 0 | type 3 and 4 (normal stools) | |||
| 2 | type 5 (soft stool) | |||
| 4 | type 6 (liquid stool, if unrelated to infection) | |||
| 6 | type 7 (watery stools) | |||
| Skin symptoms | 0 to 6 | Atopic eczema | Head-neck-trunk | Arms-legs-hands-feet |
| Absent | 0 | 0 | ||
| Mild | 1 | 1 | ||
| Moderate | 2 | 2 | ||
| Severe | 3 | 3 | ||
| 0 to 6 | Urticaria (0: no, 6: yes) | |||
| Respiratory symptoms | 0 1 2 3 | no respiratory symptoms slight symptoms mild symptoms severe symptoms | ||
| Mean | SD | Median | Min | Max | N | |
|---|---|---|---|---|---|---|
| Age (weeks) | 21.9 | 16.3 | 18.4 | 1.4 | 80.6 | 265 |
| Weight (kg) | 6.4 | 2.0 | 6.3 | 3.1 | 13.1 | 251 |
| Length (cm) | 62.7 | 8.3 | 62.5 | 37.0 | 88.6 | 231 |
| Duration of symptoms(weeks) | 12.1 | 12.9 | 7.4 | 0.0 | 64.1 | 255 |
| Age (Weeks) | ||||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | N | |
| Czech Republic | 24.1 | 14.2 | 22.6 | 1.4 | 65.1 | 84 |
| Germany | 21.4 | 17.7 | 16.4 | 3.7 | 79.4 | 36 |
| Belgium | 12.7 | 10.0 | 8.7 | 2.4 | 53.4 | 90 |
| UK | 34.1 | 17.7 | 32.1 | 4.0 | 80.6 | 55 |
| All | 21.9 | 16.3 | 18.4 | 1.4 | 80.6 | 265 ° |
| Duration of Symptoms (Weeks) | ||||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | N | |
| Czech Republic | 11.5 | 10.4 | 10.5 | 0.0 | 49.0 | 80 |
| Germany | 9.7 | 12.4 | 5.9 | 0.0 | 49.6 | 35 |
| Belgium | 6.9 | 9.1 | 4.0 | 0.0 | 56.1 | 90 |
| UK | 24.0 | 15.4 | 21.2 | 1.6 | 64.1 | 50 |
| All | 12.1 | 12.9 | 7.4 | 0.0 | 64.1 | 255 ° |
| Type of Formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SIF | pHF | eHF | AAF | Other | ||||||
| n | % | N | % | n | % | n | % | n | % | |
| Czech R | 25 | 47.2 | 19 | 35.8 | 2 | 3.8 | 5 | 9.4 | 2 | 3.8 |
| Germany | 13 | 50.0 | 9 | 34.6 | 2 | 7.7 | 2 | 7.7 | 0 | 0.0 |
| Belgium | 52 | 72.2 | 9 | 12.5 | 5 | 6.9 | 1 | 1.4 | 5 | 6.9 |
| UK | 13 | 38.2 | 2 | 5.9 | 11 | 32.4 | 4 | 11.8 | 4 | 11.8 |
| All ° | 103 | 55.7 | 39 | 21.1 | 20 | 10.8 | 12 | 6.5 | 11 | 5.9 |
| Time between Ingestion of Cow’s Milk and Onset of Symptoms (Hours) | ||||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | N | |
| Czech Republic | 219.8 | 431.4 | 60.0 | 0.1 | 2160.0 | 28 |
| Germany | 321.6 | 365.6 | 168.0 | 0.0 | 1008.0 | 10 |
| Belgium | 247.6 | 339.6 | 60.0 | 0.0 | 1080.0 | 38 |
| UK | 3.4 | 7.3 | 0.5 | 0.0 | 24.0 | 24 |
| All | 188.6 | 343.1 | 24.0 | 0.0 | 2160.0 | 100 |
| CoMiSS | ||||
|---|---|---|---|---|
| At Baseline | Final Visit | |||
| <12 | ≥12 | <12 | ≥12 | |
| n (%) | n (%) | n (%) | n (%) | |
| Czech R | 15 (17.9) | 69 (82.1) | 82 (98.8) | 1 (1.2) |
| Germany | 29 (80.6) | 7 (19.4) | 32 (97.0) | 1 (3.0) |
| Belgium | 50 (53.8) | 43 (46.2) | 87 (97.8) | 2 (2.2) |
| UK | 43 (78.2) | 12 (21.8) | 38 (92.7) | 3 (7.3) |
| All | 137 (51.1) | 131 (48.9) | 239 (97.2) | 7 (2.8) |
| Action | CoMiSS Score at First Visit | All | ||||
|---|---|---|---|---|---|---|
| <12 | ≥12 | |||||
| n | % | n | % | N | % | |
| Elimination diet mother ° | 34 | 24.8 | 62 | 47.3 | 96 | 35.8 |
| Elimination diet child * | 68 | 49.6 | 91 | 69.5 | 159 | 59.3 |
| pHF prescribed | 5 | 3.6 | 11 | 8.4 | 16 | 6.0 |
| eHF prescribed | 42 | 30.7 | 40 | 30.5 | 82 | 30.6 |
| AAF prescribed | 27 | 19.7 | 56 | 42.7 | 83 | 31.0 |
| CoMiSS at First Visit | All | |||||
|---|---|---|---|---|---|---|
| <12 | ≥12 | |||||
| n | % | n | % | N | % | |
| Czech Republic | ||||||
| Elimination diet mother | 7 | 46.7 | 34 | 49.3 | 41 | 48.8 |
| Elimination diet child | 12 | 80.0 | 53 | 76.8 | 65 | 77.4 |
| pH formula | 1 | 6.7 | 9 | 13.0 | 10 | 11.9 |
| eH formula | 0 | 0.0 | 7 | 10.1 | 7 | 8.3 |
| AA formula | 12 | 80.0 | 49 | 71.0 | 61 | 72.6 |
| Germany | ||||||
| Elimination diet mother | 5 | 17.2 | 2 | 28.6 | 7 | 19.4 |
| Elimination diet child | 12 | 41.4 | 6 | 85.7 | 18 | 50.0 |
| pH formula | 1 | 3.4 | 0 | 0.0 | 1 | 2.8 |
| eH formula | 6 | 20.7 | 4 | 57.1 | 10 | 27.8 |
| AA formula | 6 | 20.7 | 2 | 28.6 | 8 | 22.2 |
| Belgium | ||||||
| Elimination diet mother | 11 | 22.0 | 18 | 41.9 | 29 | 31.2 |
| Elimination diet child | 27 | 54.0 | 29 | 67.4 | 56 | 60.2 |
| pH formula | 3 | 6.0 | 2 | 4.7 | 5 | 5.4 |
| eH formula | 25 | 50.0 | 26 | 60.5 | 51 | 54.8 |
| AA formula | 3 | 6.0 | 5 | 11.6 | 8 | 8.6 |
| UK | ||||||
| Elimination diet mother | 11 | 25.6 | 8 | 66.7 | 19 | 34.5 |
| Elimination diet child | 17 | 39.5 | 3 | 25.0 | 20 | 36.4 |
| pH formula | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| eH formula | 11 | 25.6 | 3 | 25.0 | 14 | 25.5 |
| AA formula | 6 | 14.0 | 0 | 0.0 | 6 | 10.9 |
| Intervention | Change in CoMiSS Score at Final Visit | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Median | Min | Max | n | |
| Elimination diet mother | −5.8 | 5.0 | −6.0 | −17.0 | 12.0 | 96 |
| Partial breastfed and eHF or AAF | −10.0 | 6.2 | −11.0 | −17.0 | 0.0 | 6 |
| eHF | −6.4 | 5.1 | −6.0 | −19.0 | 5.0 | 70 |
| AAF | −9.5 | 4.5 | −10.0 | −27.0 | −1.0 | 74 |
| SPT | sIgE | |
|---|---|---|
| Czech Republic | 17.0 | 50.7 |
| Germany | 0 | 8.3 |
| Belgium | 15.1 | 31.2 |
| UK | 3.6 | 49.1 |
| Questions | Response Health Care Provider (%) | |||||
|---|---|---|---|---|---|---|
| Fully Agree | Agree | = | Disagree | Strong Disagree | ||
| 1 | The time it took you on average to complete the CoMiSS tool did NOT significantly lengthen the total consultation time. | 18 | 45 | 12 | 20 | 5 |
| 2 | Based on the experience of your use of the CoMiSS tool, you think/believe that this tool is helpful in the diagnosis of CMPA. | 25 | 61 | 9 | 5 | 0 |
| 3 | You think/believe that the tool can help physicians to diagnose infants with CMPA faster. | 17 | 64 | 13 | 6 | 0 |
| 4 | You intend to continue using the CoMiSS tool in your practice. | 21 | 49 | 18 | 9 | 3 |
| 5 | You would recommend the tool to your colleagues. | 19 | 57 | 17 | 4 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vandenplas, Y.; Belohlavkova, S.; Enninger, A.; Frühauf, P.; Makwana, N.; Järvi, A. How Are Infants Suspected to Have Cow’s Milk Allergy Managed? A Real World Study Report. Nutrients 2021, 13, 3027. https://doi.org/10.3390/nu13093027
Vandenplas Y, Belohlavkova S, Enninger A, Frühauf P, Makwana N, Järvi A. How Are Infants Suspected to Have Cow’s Milk Allergy Managed? A Real World Study Report. Nutrients. 2021; 13(9):3027. https://doi.org/10.3390/nu13093027
Chicago/Turabian StyleVandenplas, Yvan, Simona Belohlavkova, Axel Enninger, Pavel Frühauf, Niten Makwana, and Anette Järvi. 2021. "How Are Infants Suspected to Have Cow’s Milk Allergy Managed? A Real World Study Report" Nutrients 13, no. 9: 3027. https://doi.org/10.3390/nu13093027