Oxidized LDL Downregulates ABCA1 Expression via MEK/ERK/LXR Pathway in INS-1 Cells
Abstract
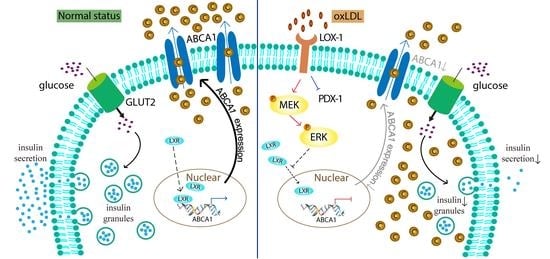
:1. Introduction
2. Materials and Methods
2.1. OxLDL and HDL
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Reverse Transcription-Quantitative, Real-Time Reverse Transcriptase-Polymerase Chain Reaction
2.5. Mutagenesis
2.6. Luciferase Reporter Gene Assay
2.7. Chromatin Immunoprecipitation (ChIP) Assay
2.8. Glucose-Stimulated Insulin Secretion (GSIS)
2.9. Cholesterol Content Assay
2.10. Oil Red O Stain
2.11. Statistical Analysis
3. Results
3.1. Glucose-Stimulated Insulin Secretion (GSIS) Was Impaired by OxLDL-Treatment in INS-1 Cells
3.2. OxLDL Induced Cholesterol Accumulation in INS-1 Cells
3.3. OxLDL Decreased the ABCA1 Expression in INS-1 Cells
3.4. OxLDL Suppressed the Transcription of ABCA1 via LOX-1/MEK/ERK Signaling Pathway in INS-1 Cells
3.5. LXR Involved in the Process of OxLDL-Suppressed ABCA1 Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Morimoto, A.; Nishimura, R.; Tajima, N. Trends in the Epidemiology of Patients with Diabetes in Japan. Jpn. Med. Assoc. J. 2010, 53, 36–40. [Google Scholar]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Njajou, O.T.; Kanaya, A.M.; Holvoet, P.; Connelly, S.; Strotmeyer, E.S.; Harris, T.B.; Cummings, S.R.; Hsueh, W.C.; Health, A.B.C.S. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab. Res. Rev. 2009, 25, 733–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okajima, F.; Kurihara, M.; Ono, C.; Nakajima, Y.; Tanimura, K.; Sugihara, H.; Tatsuguchi, A.; Nakagawa, K.; Miyazawa, T.; Oikawa, S. Oxidized but not acetylated low-density lipoprotein reduces preproinsulin mRNA expression and secretion of insulin from HIT-T15 cells. Biochim. Biophys. Acta 2005, 1687, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, B.; Vari, R.; D’Archivio, M.; Santangelo, C.; Filesi, C.; Giovannini, C.; Masella, R. Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. J. Lipid Res. 2009, 50, 832–845. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Wu, X.; Zhang, P.; Yu, Y.; Yang, M.; Jiao, P.; Wang, N.; Song, H.; Wu, Y.; Zhang, X.; et al. High-density lipoprotein inhibits Ox-Ldl-Induced adipokine secretion by upregulating SR-BI expression and suppressing ER Stress pathway. Sci. Rep. 2016, 6, 30889. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Kievit, P.; Robertson, A.K.; Kolumam, G.; Li, X.; von Wachenfeldt, K.; Valfridsson, C.; Bullens, S.; Messaoudi, I.; Bader, L.; et al. Targeting oxidized LDL improves insulin sensitivity and immune cell function in obese Rhesus macaques. Mol. Metab. 2013, 2, 256–269. [Google Scholar] [CrossRef]
- Yoshida, H.; Kondratenko, N.; Green, S.; Steinberg, D.; Quehenberger, O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem. J. 1998, 334 Pt 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Masaki, T.; Sawamura, T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: Implications in endothelial dysfunction and atherosclerosis. Pharmacol. Ther. 2002, 95, 89–100. [Google Scholar] [CrossRef]
- Yu, M.; Jiang, M.; Chen, Y.; Zhang, S.; Zhang, W.; Yang, X.; Li, X.; Li, Y.; Duan, S.; Han, J.; et al. Inhibition of Macrophage CD36 Expression and Cellular Oxidized Low Density Lipoprotein (Oxldl) Accumulation by Tamoxifen: A Peroxisome Proliferator-Activated Receptor (PPAR)gamma-Dependent Mechanism. J. Biol. Chem. 2016, 291, 16977–16989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, M.; Kanaoka, Y.; Okamoto, M.; Nakao, Y.; Inada, K.; Takayama, S.; Kataoka, Y.; Yamauchi, A. Varenicline aggravates atherosclerotic plaque formation in nicotine-pretreated ApoE knockout mice due to enhanced oxLDL uptake by macrophages through downregulation of ABCA1 and ABCG1 expression. J. Pharmacol. Sci. 2020, 142, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, M.L.J.; Walenbergh, S.M.A.; Houben, T.; Gijbels, M.J.J.; Li, J.; Hendrikx, T.; Oligschlaeger, Y.; van Gorp, P.J.; Binder, C.J.; Donners, M.; et al. Prevention of oxLDL uptake leads to decreased atherosclerosis in hematopoietic NPC1-Deficient Ldlr(-/-) mice. Atherosclerosis 2016, 255, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.P.; Grill, V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J. Clin. Endocrinol. Metab. 1995, 80, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.J.; Fielding, P.E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995, 36, 211–228. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Pape, T.D.; Timmins, J.M.; Reuwer, A.Q.; Vasanji, Z.; Marsh, B.J.; Rodrigues, B.; Johnson, J.D.; Parks, J.S.; et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007, 13, 340–347. [Google Scholar] [CrossRef]
- Brunham, L.R.; Kruit, J.K.; Verchere, C.B.; Hayden, M.R. Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Investig. 2008, 118, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Ibata, T.; Kobayashi, T.; Dong, T.; Yoshimoto, T.; Yonezaki, K.; Nagata, H.; et al. Angiotensin II induces cholesterol accumulation and impairs insulin secretion by regulating Abca1 in beta cells. J. Lipid Res. 2018, 59, 1906–1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Liao, H.; Xie, X.; Yuan, Y.; Lee, T.S.; Wang, N.; Wang, X.; Shyy, J.Y.; Stemerman, M.B. Oxidized LDL downregulates ATP-binding cassette transporter-1 in human vascular endothelial cells via inhibiting liver X receptor (LXR). Cardiovasc. Res. 2005, 68, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.C.; Lii, C.K.; Chen, H.C.; Lin, A.H.; Yang, Y.C.; Chen, H.W. Andrographolide Inhibits Oxidized LDL-Induced Cholesterol Accumulation and Foam Cell Formation in Macrophages. Am. J. Chin. Med. 2018, 46, 87–106. [Google Scholar] [CrossRef]
- Ho, C.M.; Ho, S.L.; Jeng, Y.M.; Lai, Y.S.; Chen, Y.H.; Lu, S.C.; Chen, H.L.; Chang, P.Y.; Hu, R.H.; Lee, P.H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Holvoet, P.; Lee, D.H.; Steffes, M.; Gross, M.; Jacobs, D.R., Jr. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA J. Am. Med Assoc. 2008, 299, 2287–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Hong, I.K.; Kim, B.R.; Shim, S.M.; Sung Lee, J.; Lee, H.Y.; Soo Choi, C.; Kim, B.K.; Park, T.S. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology 2015, 62, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Imachi, H.; Fukunaga, K.; Sato, S.; Kobayashi, T.; Dong, T.; Saheki, T.; Matsumoto, M.; Iwama, H.; Zhang, H.; et al. Role of ATP-binding cassette transporter A1 in suppressing lipid accumulation by glucagon-like peptide-1 agonist in hepatocytes. Mol. Metab. 2020, 34, 16–26. [Google Scholar] [CrossRef]
- Fukunaga, K.; Imachi, H.; Lyu, J.; Dong, T.; Sato, S.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Yonezaki, K.; Matsunaga, T.; et al. IGF1 suppresses cholesterol accumulation in the liver of growth hormone-deficient mice via the activation of ABCA1. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1232–E1241. [Google Scholar] [CrossRef]
- Li, J.; Murao, K.; Imachi, H.; Masugata, H.; Iwama, H.; Tada, S.; Zhang, G.X.; Kobayashi, R.; Ishida, T.; Tokumitsu, H. Exendin-4 regulates pancreatic ABCA1 transcription via Camkk/Camkiv pathway. J. Cell. Mol. Med. 2010, 14, 1083–1087. [Google Scholar] [CrossRef] [Green Version]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.A. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haefliger, J.A.; Martin, D.; Favre, D.; Petremand, Y.; Mazzolai, L.; Abderrahmani, A.; Meda, P.; Waeber, G.; Allagnat, F. Reduction of connexin36 content by ICER-1 contributes to insulin-secreting cells apoptosis induced by oxidized LDL particles. PLoS ONE 2013, 8, e55198. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Du, B.; Chen, Z. Hyperoside inhibits the effects induced by oxidized low-density lipoprotein in vascular smooth muscle cells via oxLDL-LOX-1-ERK pathway. Mol. Cell. Biochem. 2017, 433, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar] [CrossRef] [Green Version]
- Gautam, S.; Banerjee, M. The macrophage Ox-LDL receptor, CD36 and its association with type II diabetes mellitus. Mol. Genet. Metab. 2011, 102, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Karunakaran, U.; Elumalai, S.; Lee, I.K.; Lee, H.W.; Kim, Y.W.; Won, K.C. Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells. J. Diabetes Complicat. 2017, 31, 21–30. [Google Scholar] [CrossRef]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; de Courten, B.; Forbes, J.M.; et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, S.; Kloppel, G. Insulin secretion and the morphological and metabolic characteristics of pancreatic islets of hyperthyroid ob/ob mice. Endocrinology 1978, 103, 1546–1555. [Google Scholar] [CrossRef] [PubMed]
- Miyai, Y.; Murao, K.; Imachi, H.; Li, J.; Nishiuchi, Y.; Masugata, H.; Iwama, H.; Kushida, Y.; Ishida, T.; Haba, R. Exendin-4 regulates the expression of the ATP-binding cassette transporter A1 via transcriptional factor PREB in the pancreatic beta cell line. J. Endocrinol. Investig. 2011, 34, e268–e274. [Google Scholar] [CrossRef]
- Lyu, J.; Imachi, H.; Iwama, H.; Zhang, H.; Murao, K. Insulin-like Growth Factor 1 Regulates the Expression of ATP-Binding Cassette Transporter A1 in Pancreatic Beta Cells. Horm. Metab. Res. 2016, 48, 338–344. [Google Scholar] [CrossRef]
- Sato, S.; Imachi, H.; Lyu, J.; Miyai, Y.; Fukunaga, K.; Dong, T.; Ibata, T.; Kobayashi, T.; Yoshimoto, T.; Kikuchi, F.; et al. Effect of TNF-alpha on the expression of ABCA1 in pancreatic beta-cells. J. Mol. Endocrinol. 2018, 61, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Mott, S.; Yu, L.; Marcil, M.; Boucher, B.; Rondeau, C.; Genest, J., Jr. Decreased cellular cholesterol efflux is a common cause of familial hypoalphalipoproteinemia: Role of the ABCA1 gene mutations. Atherosclerosis 2000, 152, 457–468. [Google Scholar] [CrossRef]
- Koseki, M.; Matsuyama, A.; Nakatani, K.; Inagaki, M.; Nakaoka, H.; Kawase, R.; Yuasa-Kawase, M.; Tsubakio-Yamamoto, K.; Masuda, D.; Sandoval, J.C.; et al. Impaired insulin secretion in four Tangier disease patients with ABCA1 mutations. J. Atheroscler. Thromb. 2009, 16, 292–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Ketelhuth, D.F.J.; Wirstrom, T.; Ohki, T.; Forteza, M.J.; Wang, H.; Grill, V.; Wollheim, C.B.; Bjorklund, A. Increased uptake of oxLDL does not exert lipotoxic effects in insulin-secreting cells. J. Mol. Endocrinol. 2019, 62, 159–168. [Google Scholar] [CrossRef]
- Hong, D.; Bai, Y.P.; Gao, H.C.; Wang, X.; Li, L.F.; Zhang, G.G.; Hu, C.P. Ox-LDL induces endothelial cell apoptosis via the LOX-1-dependent endoplasmic reticulum stress pathway. Atherosclerosis 2014, 235, 310–317. [Google Scholar] [CrossRef]
- Duff, J.L.; Marrero, M.B.; Paxton, W.G.; Schieffer, B.; Bernstein, K.E.; Berk, B.C. Angiotensin II signal transduction and the mitogen-activated protein kinase pathway. Cardiovasc. Res. 1995, 30, 511–517. [Google Scholar] [CrossRef]
- Crews, C.M.; Alessandrini, A.; Erikson, R.L. The primary structure of MEK, a protein kinase that phosphorylates the ERK gene product. Science 1992, 258, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kakino, A.; Takeshita, H.; Hayashi, N.; Li, L.; Nakano, A.; Hanasaki-Yamamoto, H.; Fujita, Y.; Imaizumi, Y.; Toyama-Yokoyama, S.; et al. Oxidized LDL (oxLDL) activates the angiotensin II type 1 receptor by binding to the lectin-like oxLDL receptor. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3342–3356. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, Y.; Yang, X.; Sun, L.; Liu, M.; Wang, Q.; Ma, X.; Zhang, W.; Li, X.; Hu, W.; et al. Inhibition of ERK1/2 and activation of LXR synergistically reduce atherosclerotic lesions in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 948–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, V.; Wood, P.; Manetsch, M.; Darabi, M.; Cairns, R.; Hoque, M.; Chan, K.C.; Reverter, M.; Alvarez-Guaita, A.; Rye, K.A.; et al. Inhibition of mitogen-activated protein kinase Erk1/2 promotes protein degradation of ATP binding cassette transporters A1 and G1 in CHO and HuH7 cells. PLoS ONE 2013, 8, e62667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Dahlman-Wright, K. Liver X receptor in cholesterol metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Morel, E.; Demignot, S.; Chateau, D.; Chambaz, J.; Rousset, M.; Delers, F. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol. Biol. Cell 2004, 15, 132–141. [Google Scholar] [CrossRef]
- Lake, N.J.; Taylor, R.L.; Trahair, H.; Harikrishnan, K.N.; Curran, J.E.; Almeida, M.; Kulkarni, H.; Mukhamedova, N.; Hoang, A.; Low, H.; et al. TRAK2, a novel regulator of ABCA1 expression, cholesterol efflux and HDL biogenesis. Eur. Heart J. 2017, 38, 3579–3587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, T.; Lyu, J.; Imachi, H.; Kobayashi, T.; Fukunaga, K.; Sato, S.; Ibata, T.; Yoshimoto, T.; Yonezaki, K.; Iwama, H.; et al. Selective peroxisome proliferator-activated receptor-alpha modulator K-877 regulates the expression of ATP-binding cassette transporter A1 in pancreatic beta cells. Eur. J. Pharmacol. 2018, 838, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Kim, T.; Kim, H.S.; Young, M.E.; Muccio, D.D.; Atigadda, V.R.; Blum, S.I.; Tse, H.M.; Habegger, K.M.; Bhatnagar, S.; et al. A Small Molecule, UAB126, Reverses Diet-Induced Obesity and its Associated Metabolic Disorders. Diabetes 2020, 69, 2003–2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Costa, L.G.; Guizzetti, M. Retinoic acid isomers up-regulate ATP binding cassette A1 and G1 and cholesterol efflux in rat astrocytes: Implications for their therapeutic and teratogenic effects. J. Pharmacol. Exp. Ther. 2011, 338, 870–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, J.; Fukunaga, K.; Imachi, H.; Sato, S.; Kobayashi, T.; Saheki, T.; Ibata, T.; Yoshimura, T.; Iwama, H.; Murao, K. Oxidized LDL Downregulates ABCA1 Expression via MEK/ERK/LXR Pathway in INS-1 Cells. Nutrients 2021, 13, 3017. https://doi.org/10.3390/nu13093017
Lyu J, Fukunaga K, Imachi H, Sato S, Kobayashi T, Saheki T, Ibata T, Yoshimura T, Iwama H, Murao K. Oxidized LDL Downregulates ABCA1 Expression via MEK/ERK/LXR Pathway in INS-1 Cells. Nutrients. 2021; 13(9):3017. https://doi.org/10.3390/nu13093017
Chicago/Turabian StyleLyu, Jingya, Kensaku Fukunaga, Hitomi Imachi, Seisuke Sato, Toshihiro Kobayashi, Takanobu Saheki, Tomohiro Ibata, Takafumi Yoshimura, Hisakazu Iwama, and Koji Murao. 2021. "Oxidized LDL Downregulates ABCA1 Expression via MEK/ERK/LXR Pathway in INS-1 Cells" Nutrients 13, no. 9: 3017. https://doi.org/10.3390/nu13093017