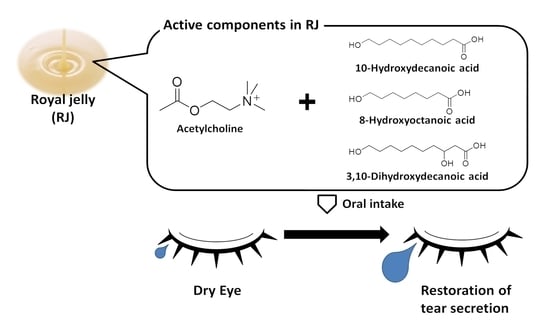
Acetylcholine and Royal Jelly Fatty Acid Combinations as Potential Dry Eye Treatment Components in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Animals
2.3. Stress-Induced Dry-Eye Model in Mice
2.4. Post-Ganglionic Denervation of the Lacrimal Gland (PGD)
2.5. Measurement of Tear Secretion in Mice
2.6. Histopathological Analysis
2.7. Acetylcholinesterase Treatment
2.8. Ex Vivo Ca2+ Imaging in the Lacrimal Gland
2.9. Analysis of Acetylcholine and RJ Fatty Acids by LC/MS/MS
2.10. Statistical Analysis
3. Results
3.1. ACh and RJ Fatty Acids Are Necessary Components of Dry-Eye Suppression in Royal Jelly
3.2. ACh with 10HDAA/8HOA/3,10DDA Preserved Tear Secretion Capacity and LG Morphology in the LG Post-Ganglionic Denervation Dry-Eye Model
3.3. 10HDAA/8HOA/3,10DDA Suppressed the Decrease of ACh-Modulated [Ca2+]i in the LG by Acetylcholinesterase Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New findings on biological actions and clinical applications of royal jelly: A review. J. Diet. Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef]
- Kajimoto, O.; Doi, S.; Matsuura, Y.; Hashimoto, K.; Nishimura, A.; Kajimoto, Y.; Hirata, H. Antihypertensive effect and safety of royal jelly protein hydrolysate in case of long-term intake for subjects with high-normal blood pressure or mild hypertension. Health Sci. 2006, 22, 204–219. [Google Scholar]
- Yamada, N.; Yoshimura, H. Effect of royal jelly on chills in Japanese young women. Nippon Eiyo Shokuryo Gakkaishi 2010, 63, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Tatefuji, T.; Asama, T.; Doi, S.; Sugano, T.; Hashimoto, K. Efficacy of royal jelly supplement on neck muscle strain (Japanese: Katakori) in healthy adult women: A randomized double-blind, placebo-controlled trial. East. Med. 2010, 26, 55–64. [Google Scholar]
- Asama, T.; Matsuzaki, H.; Fukushima, S.; Tatefuji, T.; Hashimoto, K.; Takeda, T. Royal jelly supplementation improves menopausal symptoms such as backache, low back pain, and anxiety in postmenopausal Japanese women. Evid. Based Complementary Altern. Med. 2018, 2018, 4868412. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Jazayeri, S.; Khoshpay, B.; Malek, M.; Hosseini, A.F.; Hosseini, S.; Shidfar, F. Royal jelly decreases blood pressure, serum glucose, and interleukin-6 in patients with type 2 diabetes on an iso-caloric diet. J. Nutr. Food Secur. 2017, 2, 300–307. [Google Scholar]
- Meng, G.; Wang, H.; Pei, Y.; Li, Y.; Wu, H.; Song, Y.; Guo, Q.; Guo, H.; Fukushima, S.; Tatefuji, T.; et al. Effects of protease-treated royal jelly on muscle strength in elderly nursing home residents: A randomized, double-blind, placebo-controlled, dose-response study. Sci. Rep. 2017, 7, 11416. [Google Scholar] [CrossRef] [Green Version]
- Asama, T.; Okumura, N.; Yamaki, A.; Okuma, A.; Numano, K. Effect of protease-digested royal jelly supplementation on skin conditions and safety in healthy Japanese adults-A randomized, double-blind, placebo-controlled, parallel-group comparison study. Jpn. Pharmacol. Ther. 2020, 48, 79–88. [Google Scholar]
- Sugiyama, T.; Takahashi, K.; Mori, H. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, as a modulator of the innate immune responses. Endocr. Metab. Immune Disord. Drug Targets 2012, 12, 368–376. [Google Scholar] [CrossRef]
- Terada, Y.; Narukawa, M.; Watanabe, T. Specific hydroxy fatty acids in royal jelly activate TRPA1. J. Agric. Food Chem. 2011, 59, 2627–2635. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takikawa, M.; Kumagai, A.; Hirata, H.; Soga, M.; Yamashita, Y.; Ueda, M.; Ashida, H.; Tsuda, T. 10-Hydroxy-2-decenoic acid, a unique medium-chain fatty acid, activates 5′-AMP-activated protein kinase in L6 myotubes and mice. Mol. Nutr. Food Res. 2013, 57, 1794–1802. [Google Scholar] [CrossRef]
- Gu, L.; Zeng, H.; Maeda, K. 10-Hydroxy-2-decenoic acid in royal jelly extract induced both filaggrin and amino acid in a cultured human three-dimensional epidermis model. Cosmetics 2017, 4, 4040048. [Google Scholar] [CrossRef] [Green Version]
- Isidorov, V.A.; Bakier, S.; Grzech, I. Gas chromatographic-mass spectrometric investigation of volatile and extractable compounds of crude royal jelly. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 885–886, 109–116. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Wang, T.; Yang, L.; He, Y.Y.; Miskimins, K.; Qian, S.Y. Knockdown delta-5-desaturase in breast cancer cells that overexpress COX-2 results in inhibition of growth, migration and invasion via a dihomo-γ-linolenic acid peroxidation dependent mechanism. BMC Cancer 2018, 18, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isidorow, W.; Witkowski, S.; Iwaniuk, P.; Zambrzycka, M.; Swiecicka, I. Royal jelly aliphatic acids contribute to antimicrobial activity of honey. J. Apic. Sci. 2018, 62, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [Green Version]
- Tubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Calonge, M. The treatment of dry eye. Surv. Ophthalmol. 2001, 45, S227–S239. [Google Scholar] [CrossRef]
- Inoue, S.; Kawashima, M.; Hisamura, R.; Imada, T.; Izuta, Y.; Nakamura, S.; Ito, M.; Tsubota, K. Clinical evaluation of a royal jelly supplementation for the restoration of dry eye: A prospective randomized double blind placebo controlled study and an experimental mouse model. PLoS ONE 2017, 12, e0169069. [Google Scholar] [CrossRef] [PubMed]
- Imada, T.; Nakamura, S.; Kitamura, N.; Shibuya, I.; Tsubota, K. Oral administration of royal jelly restores tear secretion capacity in rat blink-suppressed dry eye model by modulating lacrimal gland function. PLoS ONE 2014, 9, e106338. [Google Scholar] [CrossRef]
- Wei, W.; Wei, M.; Kang, X.; Deng, H.; Lu, Z. A novel method developed for acetylcholine detection in royal jelly by using capillary electrophoresis coupled with electrogenerated chemiluminescence based on a simple reaction. Electrophoresis 2009, 30, 1949–1952. [Google Scholar] [CrossRef]
- Moriyama, T.; Yanagihara, M.; Yano, E.; Kimura, G.; Seishima, M.; Tani, H.; Kanno, T.; Nakamura-Hirota, T.; Hashimoto, K.; Tatefuji, T.; et al. Hypoallergenicity and immunological characterization of enzyme-treated royal jelly from Apis mellifera. Biosci. Biotechnol. Biochem. 2013, 77, 789–795. [Google Scholar] [CrossRef]
- Kodai, T.; Nakatani, T.; Noda, N. The absolute configurations of hydroxy fatty acids from the royal jelly of honeybees (Apis mellifera). Lipids 2011, 46, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Tani, H.; Takahashi, S.; Hasumi, K.; Tatefuji, T.; Hongo, Y.; Koshino, H. Isolation of (E)-9,10-dihydroxy-2-decenoic acid from royal jelly and determination of the absolute configuration by chemical synthesis. Tetrahedron Asymmetry 2009, 20, 457–460. [Google Scholar] [CrossRef]
- Misumi, S.; Ikuta, T.; Tatefuji, T.; Tani, H.; Inooka, H. Mucosal Immunomodulatory. U.S. Patent Publication No. US2019/0230967 A1, 1 August 2019. [Google Scholar]
- Yamaga, M.; Tani, H.; Yamaki, A.; Tatefuji, T.; Hashimoto, K. Metabolism and pharmacokinetics of medium chain fatty acids after oral administration of royal jelly to healthy subjects. RSC Adv. 2019, 9, 15392–15401. [Google Scholar] [CrossRef] [Green Version]
- Imada, T.; Nakamura, S.; Hisamura, R.; Izuta, Y.; Oshima, Y.; Nemoto, T.; Tsubota, K. In vivo imaging of Ca2+ dynamics in lacrimal gland of Yellow Cameleon-Nano transgenic mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5658. [Google Scholar]
- Sano, K.; Kawashima, M.; Imada, T.; Suzuki, T.; Nakamura, S.; Mimura, M.; Tanaka, K.F.; Tsubota, K. Enriched environment alleviates stress-induced dry-eye through the BDNF axis. Sci. Rep. 2019, 9, 3422. [Google Scholar] [CrossRef]
- Jin, K.; Imada, T.; Hisamura, R.; Ito, M.; Toriumi, H.; Tanaka, K.F.; Nakamura, S.; Tsubota, K. Identification of lacrimal gland postganglionic innervation and its regulation of tear secretion. Am. J. Pathol. 2020, 190, 1068–1079. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worek, F.; Mast, U.; Kiderlen, D.; Diepold, C.; Eyer, P. Improved determination of acetylcholinesterase activity in human whole blood. Clin. Chem. Acta 1999, 288, 73–90. [Google Scholar] [CrossRef]
- Sine, J.P.; Ferrand, R.; Cloarec, D.; Lehur, P.A.; Colas, B. Human intestine epithelial cell acetyl- and butyrylcholinesterase. Mol. Cell Biochem. 1991, 108, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Guardia de Souza E Silva, T.; do Val de Paulo, M.E.F.; da Silva, J.R.M.; da Silva Alves, A.; Britto, L.R.G.; Xavier, G.F.; Lopes Sandoval, M.R. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon 2020, 6, e03281. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shidfar, F.; Jazayeri, S.; Mousavi, S.N.; Malek, M.; Hosseini, A.F.; Khoshpey, B. Does supplementation with royal jelly improve oxidative stress and insulin resistance in type 2 diabetic patients? Iran J. Public Health 2015, 44, 797–803. [Google Scholar] [PubMed]
- Gwynne, R.M.; Thomas, E.A.; Goh, S.M.; Sjövall, H.; Bornstein, J.C. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. J. Physiol. 2004, 556 Pt 2, 557–569. [Google Scholar] [CrossRef]
- Baenziger, J.E.; Hénault, C.M.; Therien, J.P.D.; Sun, J. Nicotinic acetylcholine receptor-lipid interactions: Mechanistic insight and biological function. Biochim. Biophys. Acta 2015, 1848, 1806–1817. [Google Scholar] [CrossRef] [Green Version]
- Jakubík, J.; Tucek, S.; El-Fakahany, E.E. Role of receptor protein and membrane lipids in xanomeline wash-resistant binding to muscarinic M1 receptors. J. Pharmacol. Exp. Ther. 2004, 308, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Compounds | (%) 1 | Oral Administration Dosage (mg/kg) 2 | Source or Reference |
|---|---|---|---|
| acetylcholine (ACh) | 0.023 | 0.069 | Tokyo Chemical (Tokyo, Japan) |
| 8-hydroxyoctanoic acid (8HOA) | 0.312 | 0.936 | Sigma-Aldrich (St. Louis, MO, USA) |
| (R)-3,10-dihydroxydecanoic acid (3,10DDA) | 0.363 | 1.089 | Isolation from RJ according to the previous report (Noda et al. [25]). |
| 10-hydroxydecanoic acid (10HDAA) | 1.347 | 4.041 | Combi-Blocks (San Diego, CA, USA) |
| (E)-9,10-dihydroxy-2-decenoic acid (9,10D2DA) | 0.001 | 0.003 | Prepared by the mixing of synthetic (R) and (S)-acids in a ratio of R/S = 3.5/1 (Tani et al. [26]) |
| (E)-10-hydroxy-2-decenoic acid (10H2DA) | 4.267 | 12.801 | Hangzhou Eastbiopharm (Hangzhou, China) |
| (E)-2-decenedioic acid (2DA) | 0.435 | 1.305 | Sundia MediTech (Shanghai, China) |
| sebacic acid (SA) | 0.279 | 0.837 | Sigma-Aldrich (St. Louis, MO, USA) |
| (E,R)-11,12-dihydroxy-2-dodecenoic acid (11,12D2DA) | 0.001 | 0.003 | Prepared according to the procedures described in the patent [27] and Supporting Information (Scheme S1). Analytical data are provided in the Supporting Information (Figures S1 and S2). |
| 12-hydroxydodecanoic acid (12HDA) | 0.049 | 0.147 | MP Biomedicals (Santa Ana, CA, USA) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaga, M.; Imada, T.; Tani, H.; Nakamura, S.; Yamaki, A.; Tsubota, K. Acetylcholine and Royal Jelly Fatty Acid Combinations as Potential Dry Eye Treatment Components in Mice. Nutrients 2021, 13, 2536. https://doi.org/10.3390/nu13082536
Yamaga M, Imada T, Tani H, Nakamura S, Yamaki A, Tsubota K. Acetylcholine and Royal Jelly Fatty Acid Combinations as Potential Dry Eye Treatment Components in Mice. Nutrients. 2021; 13(8):2536. https://doi.org/10.3390/nu13082536
Chicago/Turabian StyleYamaga, Masayuki, Toshihiro Imada, Hiroko Tani, Shigeru Nakamura, Ayanori Yamaki, and Kazuo Tsubota. 2021. "Acetylcholine and Royal Jelly Fatty Acid Combinations as Potential Dry Eye Treatment Components in Mice" Nutrients 13, no. 8: 2536. https://doi.org/10.3390/nu13082536