Caffeine during High-Intensity Whole-Body Exercise: An Integrative Approach beyond the Central Nervous System
Abstract
:1. Introduction
2. The Pulmonary System
3. The Cardiovascular System
4. The Skeletal Muscle
5. Connection between Peripheral and Central Nervous Systems
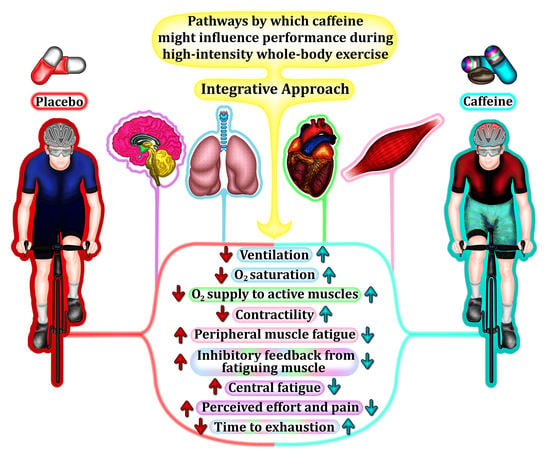
6. An Integrative Approach to Caffeine Effects during High-Intensity Whole-Body Exercise
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desbrow, B.; Leveritt, M. Awareness and use of caffeine by athletes competing at the 2005 Ironman Triathlon World Championships. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Spriet, L.L. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J. Appl. Physiol. 1995, 78, 867–874. [Google Scholar] [CrossRef]
- Ståhle, L.; Segersvärd, S.; Ungerstedt, U. Drug distribution studies with microdialysis II. Caffeine and theophylline in blood, brain and other tissues in rats. Life Sci. 1991, 49, 1843–1852. [Google Scholar] [CrossRef]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance—An umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef]
- Spineli, H.; Pinto, M.P.; Dos Santos, B.P.; Lima-Silva, A.E.; Bertuzzi, R.; Gitaí, D.L.G.; de Araujo, G.G. Caffeine improves various aspects of athletic performance in adolescents independent of their 163 C > A CYP1A2 genotypes. Scand. J. Med. Sci. Sports 2020, 30, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.; Barrett, L.A.; Chow, J.Y.; Burns, S.F. Effects of Caffeine Supplementation on Performance in Ball Games. Sports Med. 2017, 47, 2453–2471. [Google Scholar] [CrossRef]
- Southward, K.; Rutherfurd-Markwick, K.J.; Ali, A. The Effect of Acute Caffeine Ingestion on Endurance Performance: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1913–1928. [Google Scholar] [CrossRef]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.G.; Brooks, M.B.; Cincotta, J.; Manjourides, J.D. Establishing a relationship between the effect of caffeine and duration of endurance athletic time trial events: A systematic review and meta-analysis. J. Sci. Med. Sport 2019, 22, 232–238. [Google Scholar] [CrossRef]
- Doherty, M.; Smith, P.M. Effects of caffeine ingestion on exercise testing: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 626–646. [Google Scholar] [CrossRef]
- Weavil, J.C.; Amann, M. Neuromuscular fatigue during whole body exercise. Curr. Opin. Physiol. 2019, 10, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Vanhatalo, A.; Burnley, M.; Morton, R.H.; Poole, D.C. Critical power: Implications for determination of VO2max and exercise tolerance. Med. Sci. Sports Exerc. 2010, 42, 1876–1890. [Google Scholar] [CrossRef]
- Silveira, R.; Andrade-Souza, V.A.; Arcoverde, L.; Tomazini, F.; Sansonio, A.; Bishop, D.J.; Bertuzzi, R.; Lima-Silva, A.E. Caffeine Increases Work Done above Critical Power, but Not Anaerobic Work. Med. Sci. Sports Exerc. 2018, 50, 131–140. [Google Scholar] [CrossRef]
- Salvatore, C.A.; Jacobson, M.A.; Taylor, H.E.; Linden, J.; Johnson, R.G. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10365–10369. [Google Scholar] [CrossRef] [Green Version]
- Lynge, J.; Hellsten, Y. Distribution of adenosine A1, A2A and A2B receptors in human skeletal muscle. Acta Physiol. Scand. 2000, 169, 283–290. [Google Scholar] [CrossRef]
- Morales, A.P.; Sampaio-Jorge, F.; Barth, T.; Pierucci, A.P.T.R.; Ribeiro, B.G. Caffeine Supplementation for 4 Days Does Not Induce Tolerance to the Ergogenic Effects Promoted by Acute Intake on Physiological, Metabolic, and Performance Parameters of Cyclists: A Randomized, Double-Blind, Crossover, Placebo-Controlled Study. Nutrients 2020, 12, 2101. [Google Scholar] [CrossRef]
- Chapman, R.F.; Mickleborough, T.D. The effects of caffeine on ventilation and pulmonary function during exercise: An often-overlooked response. Phys. Sportsmed. 2009, 37, 97–103. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Amann, M.; Romer, L.M.; Miller, J.D. Respiratory system determinants of peripheral fatigue and endurance performance. Med. Sci. Sports Exerc. 2008, 40, 457–461. [Google Scholar] [CrossRef]
- Amann, M. Pulmonary system limitations to endurance exercise performance in humans. Exp. Physiol. 2012, 97, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, J.A.; Wagner, P.D. Exercise-induced arterial hypoxemia. J. Appl. Physiol. 1999, 87, 1997–2006. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Hanson, P.G.; Henderson, K.S. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J. Physiol. 1984, 355, 161–175. [Google Scholar] [CrossRef]
- Harms, C.A.; McClaran, S.R.; Nickele, G.A.; Pegelow, D.F.; Nelson, W.B.; Dempsey, J.A. Exercise-induced arterial hypoxaemia in healthy young women. J. Physiol. 1998, 507, 619–628. [Google Scholar] [CrossRef]
- Romer, L.M.; Dempsey, J.A. Effects of exercise-induced arterial hypoxaemia on limb muscle fatigue and performance. Clin. Exp. Pharmacol. Physiol. 2006, 33, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Dominelli, P.B.; Sheel, A.W. Exercise-induced arterial hypoxemia; some answers, more questions. Appl. Physiol. Nutr. Metab. 2019, 44, 571–579. [Google Scholar] [CrossRef]
- Harms, C.A.; Babcock, M.A.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Dempsey, J.A. Respiratory muscle work compromises leg blood flow during maximal exercise. J. Appl. Physiol. 1997, 82, 1573–1583. [Google Scholar] [CrossRef] [Green Version]
- Harms, C.A.; Stager, J.M. Low chemoresponsiveness and inadequate hyperventilation contribute to exercise-induced hypoxemia. J. Appl. Physiol. 1995, 79, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Wetter, T.J.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Hanson, P.; Dempsey, J.A. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J. Appl. Physiol. 1998, 85, 609–618. [Google Scholar] [CrossRef]
- Chapman, R.F.; Stager, J.M. Caffeine stimulates ventilation in athletes with exercise-induced hypoxemia. Med. Sci. Sports Exerc. 2008, 40, 1080–1086. [Google Scholar] [CrossRef]
- Trippenbach, T.; Zinman, R.; Milic-Emili, J. Caffeine effect on breathing pattern and vagal reflexes in newborn rabbits. Respir. Physiol. 1980, 40, 211–225. [Google Scholar] [CrossRef]
- Richmond, G.H. Action of Caffeine and Aminophylline as Respiratory Stimulants in Man. J. Appl. Physiol. 1949, 2, 16–23. [Google Scholar] [CrossRef]
- D’Urzo, A.D.; Jhirad, R.; Jenne, H.; Avendano, M.A.; Rubinstein, I.; D’Costa, M.; Goldstein, R.S. Effect of caffeine on ventilatory responses to hypercapnia, hypoxia, and exercise in humans. J. Appl. Physiol. 1990, 68, 322–328. [Google Scholar] [CrossRef]
- Duffy, P.; Phillips, Y.Y. Caffeine consumption decreases the response to bronchoprovocation challenge with dry gas hyperventilation. Chest 1991, 99, 1374–1377. [Google Scholar] [CrossRef] [Green Version]
- Kivity, S.; Aharon, Y.B.; Man, A.; Topilsky, M. The Effect of Caffeine on Exercise-Induced Bronchoconstriction. Chest 1990, 97, 1083–1085. [Google Scholar] [CrossRef]
- Merlini, M.; Beato, M.; Marcora, S.; Dickinson, J. The Effect of 1600 μg Inhaled Salbutamol Administration on 30 m SprInt. Performance Pre and Post a Yo-Yo Intermittent Running Test in Football Players. J. Sports Sci. Med. 2019, 18, 716–721. [Google Scholar]
- Molphy, J.; Dickinson, J.W.; Chester, N.J.; Loosemore, M.; Whyte, G. The Effect of 400 µg Inhaled Salbutamol on 3 km Time Trial Performance in a Low Humidity Environment. J. Sports Sci. Med. 2017, 16, 581–588. [Google Scholar]
- Supinski, G.S.; Levin, S.; Kelsen, S.G. Caffeine effect on respiratory muscle endurance and sense of effort during loaded breathing. J. Appl. Physiol. 1986, 60, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Miyamara, M.; Honda, Y. Oxygen intake and cardiac output during maximal treadmill and bicycle exercise. J. Appl. Physiol. 1972, 32, 185–188. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Louvaris, Z.; Wagner, P.D. Respiratory and locomotor muscle blood flow during exercise in health and chronic obstructive pulmonary disease. Exp. Physiol. 2020, 105, 1990–1996. [Google Scholar] [CrossRef]
- Stark-Leyva, K.N.; Beck, K.C.; Johnson, B.D. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J. Appl. Physiol. 2004, 96, 1920–1927. [Google Scholar] [CrossRef]
- Sung, B.H.; Lovallo, W.R.; Pincomb, G.A.; Wilson, M.F. Effects of caffeine on blood pressure response during exercise in normotensive healthy young men. Am. J. Cardiol. 1990, 65, 909–913. [Google Scholar] [CrossRef]
- Bunsawat, K.; White, D.W.; Kappus, R.M.; Baynard, T. Caffeine delays autonomic recovery following acute exercise. Eur. J. Prev. Cardiol. 2015, 22, 1473–1479. [Google Scholar] [CrossRef]
- Olcina, G.J.; Muñoz, D.; Timón, R.; Caballero, M.J.; Maynar, J.I.; Córdova, A.; Maynar, M. Effect of caffeine on oxidative stress during maximum incremental exercise. J. Sports Sci. Med. 2006, 5, 621–628. [Google Scholar]
- Crowe, M.J.; Leicht, A.S.; Spinks, W.L. Physiological and cognitive responses to caffeine during repeated, high-intensity exercise. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 528–544. [Google Scholar] [CrossRef]
- Vanakoski, J.; Kosunen, V.; Meririnne, E.; Seppälä, T. Creatine and caffeine in anaerobic and aerobic exercise: Effects on physical performance and pharmacokinetic considerations. Int. J. Clin. Pharmacol. Ther. 1998, 36, 258–262. [Google Scholar]
- Ruíz-Moreno, C.; Lara, B.; Brito de Souza, D.; Gutiérrez-Hellín, J.; Romero-Moraleda, B.; Cuéllar-Rayo, Á.; Del Coso, J. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br. J. Clin. Pharmacol. 2020, 86, 861–867. [Google Scholar] [CrossRef]
- Zhou, B.; Conlee, R.K.; Jensen, R.; Fellingham, G.W.; George, J.D.; Fisher, A.G. Stroke volume does not plateau during graded exercise in elite male distance runners. Med. Sci. Sports Exerc. 2001, 33, 1849–1854. [Google Scholar] [CrossRef]
- Secher, N.H.; Volianitis, S. Are the arms and legs in competition for cardiac output? Med. Sci. Sports Exerc. 2006, 38, 1797–1803. [Google Scholar] [CrossRef]
- Klausen, K.; Secher, N.H.; Clausen, J.P.; Hartling, O.; Trap-Jensen, J. Central and regional circulatory adaptations to one-leg training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 52, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Arend, L.J.; Haramati, A.; Thompson, C.I.; Spielman, W.S. Adenosine-induced decrease in renin release: Dissociation from hemodynamic effects. Am. J. Physiol. 1984, 247, F447–F452. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.W.; Molé, P.A.; Shaffrath, J.D.; Stebbins, C.L. Effects of caffeine on blood pressure, heart rate, and forearm blood flow during dynamic leg exercise. J. Appl. Physiol. 1998, 85, 154–159. [Google Scholar] [CrossRef] [Green Version]
- Mira, J.; Floreani, M.; Savoldelli, A.; Amery, K.; Koral, J.; Oranchuk, D.J.; Messonnier, L.; Rupp, T.; Millet, G.Y. Neuromuscular Fatigue of Cycling Exercise in Hypoxia. Med. Sci. Sports Exerc. 2020. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.; Cafarelli, E. Caffeine increases time to fatigue by maintaining force and not by altering firing rates during submaximal isometric contractions. J. Appl. Physiol. 2005, 99, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Pethick, J.; Winter, S.L.; Burnley, M. Caffeine ingestion attenuates fatigue-induced loss of muscle torque complexity. Med. Sci. Sports Exerc. 2018, 50, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.M.; Aubier, M.; Jardim, J.; Aranda, J.V.; Macklem, P.T. Effect of caffeine on skeletal muscle function before and after fatigue. J. Appl. Physiol. 1983, 54, 1303–1305. [Google Scholar] [CrossRef]
- Mohr, T.; Van Soeren, M.; Graham, T.E.; Kjaer, M. Caffeine ingestion and metabolic responses of tetraplegic humans during electrical cycling. J. Appl. Physiol. 1998, 85, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarnopolsky, M.; Cupido, C. Caffeine potentiates low frequency skeletal muscle force in habitual and nonhabitual caffeine consumers. J. Appl. Physiol. 2000, 89, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, D.; Cheng, A.J.; Donnelly, C.; Bourdillon, N.; Gassner, A.-L.; Geiser, L.; Rudaz, S.; Kayser, B.; Westerblad, H.; Place, N. Toxic doses of caffeine are needed to increase skeletal muscle contractility. Am. J. Physiol. Cell Physiol. 2019, 316, C246–C251. [Google Scholar] [CrossRef]
- Tallis, J.; Duncan, M.J.; James, R.S. What can isolated skeletal muscle experiments tell us about the effects of caffeine on exercise performance? Br. J. Pharmacol. 2015, 172, 3703–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallis, J.; James, R.S.; Cox, V.M.; Duncan, M.J. The effect of physiological concentrations of caffeine on the power output of maximally and submaximally stimulated mouse EDL (fast) and soleus (slow) muscle. J. Appl. Physiol. 2012, 112, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Tallis, J.; Higgins, M.F.; Cox, V.M.; Duncan, M.J.; James, R.S. An exercise-induced improvement in isolated skeletal muscle contractility does not affect the performance-enhancing benefit of 70 µmol l–1 caffeine treatment. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [Green Version]
- Felippe, L.C.; Ferreira, G.A.; Learsi, S.K.; Boari, D.; Bertuzzi, R.; Lima-Silva, A.E. Caffeine increases both total work performed above critical power and peripheral fatigue during a 4-km cycling time trial. J. Appl. Physiol. 2018, 124, 1491–1501. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M.J.; Minahan, C.L.; Sabapathy, S. Caffeine improves supramaximal cycling but not the rate of anaerobic energy release. Eur. J. Appl. Physiol. 2010, 109, 287–295. [Google Scholar] [CrossRef]
- Mohr, M.; Nielsen, J.J.; Bangsbo, J. Caffeine intake improves intense intermittent exercise performance and reduces muscle interstitial potassium accumulation. J. Appl. Physiol. 2011, 111, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Chesley, A.; Howlett, R.A.; Heigenhauser, G.J.; Hultman, E.; Spriet, L.L. Regulation of muscle glycogenolytic flux during intense aerobic exercise after caffeine ingestion. Am. J. Physiol. 1998, 275, R596–R603. [Google Scholar] [CrossRef]
- Wilson, L.B.; Andrew, D.; Craig, A.D. Activation of spinobulbar lamina I neurons by static muscle contraction. J. Neurophysiol. 2002, 87, 1641–1645. [Google Scholar] [CrossRef] [Green Version]
- Wilson, L.B.; Hand, G.A. The pressor reflex evoked by static contraction: Neurochemistry at the site of the first synapse. Brain Res. 1997, 23, 196–209. [Google Scholar] [CrossRef]
- Blain, G.M.; Mangum, T.S.; Sidhu, S.K.; Weavil, J.C.; Hureau, T.J.; Jessop, J.E.; Bledsoe, A.D.; Richardson, R.S.; Amann, M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J. Physiol. 2016, 594, 5303–5315. [Google Scholar] [CrossRef] [Green Version]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011, 589, 5299–5309. [Google Scholar] [CrossRef]
- Amann, M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J. Physiol. 2009, 587, 271–283. [Google Scholar] [CrossRef]
- Amann, M. Significance of Group III and IV muscle afferents for the endurance exercising human. Clin. Exp. Pharmacol. Physiol. 2012, 39, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Hureau, T.J.; Romer, L.M.; Amann, M. The ‘sensory tolerance limit’: A hypothetical construct determining exercise performance? Eur. J. Sport Sci. 2018, 18, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine receptors and the central nervous system. In Adenosine Receptors in Health and Disease; Wilson, C., Mustafa, S., Eds.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 471–534. [Google Scholar]
- Sawynok, J. Adenosine receptor activation and nociception. Eur. J. Pharmacol. 1998, 347, 1–11. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Mohr, M.; Fulford, J.; Jackman, S.R.; Ermidis, G.; Krustrup, P.; Mileva, K.N. Improved exercise tolerance with caffeine is associated with modulation of both peripheral and central neural processes in human participants. Front. Nutr. 2018, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Kalmar, J.M.; Cafarelli, E. Central fatigue and transcranial magnetic stimulation: Effect of caffeine and the confound of peripheral transmission failure. J. Neurosci. Methods 2004, 138, 15–26. [Google Scholar] [CrossRef]
- Kalmar, J.M.; Cafarelli, E. Central excitability does not limit postfatigue voluntary activation of quadriceps femoris. J. Appl. Physiol. 2006, 100, 1757–1764. [Google Scholar] [CrossRef] [Green Version]
- Walton, C.; Kalmar, J.; Cafarelli, E. Caffeine increases spinal excitability in humans. Muscle Nerve 2003, 28, 359–364. [Google Scholar] [CrossRef]
- Black, C.D.; Waddell, D.E.; Gonglach, A.R. Caffeine’s Ergogenic Effects on Cycling: Neuromuscular and Perceptual Factors. Med. Sci. Sports Exerc. 2015, 47, 1145–1158. [Google Scholar] [CrossRef]
- Elmenhorst, D.; Meyer, P.T.; Matusch, A.; Winz, O.H.; Bauer, A. Caffeine occupancy of human cerebral A1 adenosine receptors: In vivo quantification with 18F-CPFPX and PET. J. Nucl. Med. 2012, 53, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, R.N.O.; Cronin, N.J.; Kyröläinen, H.; Hintikka, J.; Avela, J. Effects of caffeine on neuromuscular function in a non-fatigued state and during fatiguing exercise. Exp. Physiol. 2020, 105, 690–706. [Google Scholar] [CrossRef]
- Merton, P. Voluntary strength and fatigue. J. Physiol. 1954, 123, 553–564. [Google Scholar] [CrossRef]
- Motl, R.W.; O’Connor, P.J.; Tubandt, L.; Puetz, T.; Ely, M.R. Effect of caffeine on leg muscle pain during cycling exercise among females. Med. Sci. Sports Exerc. 2006, 38, 598–604. [Google Scholar] [CrossRef]
- Astorino, T.A.; Terzi, M.N.; Roberson, D.W.; Burnett, T.R. Effect of caffeine intake on pain perception during high-intensity exercise. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 27–32. [Google Scholar] [CrossRef]
- Gliottoni, R.C.; Motl, R.W. Effect of caffeine on leg-muscle pain during intense cycling exercise: Possible role of anxiety sensitivity. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 103–115. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Silva, A.E.; Cristina-Souza, G.; Silva-Cavalcante, M.D.; Bertuzzi, R.; Bishop, D.J. Caffeine during High-Intensity Whole-Body Exercise: An Integrative Approach beyond the Central Nervous System. Nutrients 2021, 13, 2503. https://doi.org/10.3390/nu13082503
Lima-Silva AE, Cristina-Souza G, Silva-Cavalcante MD, Bertuzzi R, Bishop DJ. Caffeine during High-Intensity Whole-Body Exercise: An Integrative Approach beyond the Central Nervous System. Nutrients. 2021; 13(8):2503. https://doi.org/10.3390/nu13082503
Chicago/Turabian StyleLima-Silva, Adriano E., Gislaine Cristina-Souza, Marcos D. Silva-Cavalcante, Romulo Bertuzzi, and David J. Bishop. 2021. "Caffeine during High-Intensity Whole-Body Exercise: An Integrative Approach beyond the Central Nervous System" Nutrients 13, no. 8: 2503. https://doi.org/10.3390/nu13082503