The Clinical Value of Nutritional Care before and during Active Cancer Treatment
Abstract
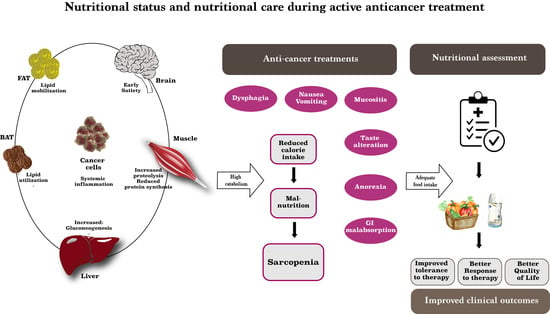
:1. Introduction
2. Detecting Malnutrition from the Very Beginning of Cancer Care: Who Starts Well Is Half the Battle
3. Nutritional Care during Active Chemotherapy: Any New Tricks for This Old Issue?
4. Nutritional Care during Immunotherapy: Does the Boundless Prairie Have a Fence?
5. Nutritional Care during Later Treatment Lines and Beyond: Is the Final Hurdle Worth Jumping?
6. Nutritional Care during Cancer Treatment: The Changing Role of Oncologists, Nutritionists and Nurses
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, B.H.L.; Birdsell, L.A.; Martin, L.; Baracos, V.E.; Fearon, K.C.H. Sarcopenia in an Overweight or Obese Patient Is an Adverse Prognostic Factor in Pancreatic Cancer. Clin. Cancer Res. 2009, 15, 6973–6979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, W.J.; Campbell, W.W. Sarcopenia and Age-Related Changes in Body Composition and Functional Capacity. J. Nutr. 1993, 123, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, Risk Factors and Clinical Implications of Malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, H.; Isenring, E.; Yates, P. The Prevalence of Nutrition Impact Symptoms and Their Relationship to Quality of Life and Clinical Outcomes in Medical Oncology Patients. Support. Care Cancer 2009, 17, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Kim, S.-E.; Kang, Y.-K.; Ryoo, B.-Y.; Ryu, M.-H.; Jeong, J.H.; Kang, S.S.; Yang, M.; Lee, J.E.; Sung, M.-K. Association of Nutritional Status-Related Indices and Chemotherapy-Induced Adverse Events in Gastric Cancer Patients. BMC Cancer 2016, 16, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellrich, N.-C.; Handschel, J.; Holtmann, H.; Krüskemper, G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef] [PubMed]
- Planas, M.; Álvarez-Hernández, J.; León-Sanz, M.; Celaya-Pérez, S.; Araujo, K.; García de Lorenzo, A.; PREDyCES® Researchers. Prevalence of Hospital Malnutrition in Cancer Patients: A Sub-Analysis of the PREDyCES® Study. Support. Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basile, D.; Parnofiello, A.; Vitale, M.G.; Cortiula, F.; Gerratana, L.; Fanotto, V.; Lisanti, C.; Pelizzari, G.; Ongaro, E.; Bartoletti, M.; et al. The IMPACT Study: Early Loss of Skeletal Muscle Mass in Advanced Pancreatic Cancer Patients. J. Cachexia Sarcopenia Muscle 2019, 10, 368–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébuterne, X.; Lemarié, E.; Michallet, M.; de Montreuil, C.B.; Schneider, S.M.; Goldwasser, F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients with Cancer. JPEN J. Parenter. Enter. Nutr. 2014, 38, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Pardo, J.; Jara, C.; Zugazabeitia, L.; Carulla, J.; de Las Peñas, R.; García-Cabrera, E.; Luz Azuara, M.; Casadó, J.; Gómez-Candela, C. An Epidemiological Evaluation of the Prevalence of Malnutrition in Spanish Patients with Locally Advanced or Metastatic Cancer. Clin. Nutr. 2005, 24, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Attar, A.; Malka, D.; Sabaté, J.M.; Bonnetain, F.; Lecomte, T.; Aparicio, T.; Locher, C.; Laharie, D.; Ezenfis, J.; Taieb, J. Malnutrition Is High and Underestimated during Chemotherapy in Gastrointestinal Cancer: An AGEO Prospective Cross-Sectional Multicenter Study. Nutr. Cancer 2012, 64, 535–542. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [Green Version]
- Aapro, M.; Arends, J.; Bozzetti, F.; Fearon, K.; Grunberg, S.M.; Herrstedt, J.; Hopkinson, J.; Jacquelin-Ravel, N.; Jatoi, A.; Kaasa, S.; et al. Early Recognition of Malnutrition and Cachexia in the Cancer Patient: A Position Paper of a European School of Oncology Task Force. Ann. Oncol. 2014, 25, 1492–1499. [Google Scholar] [CrossRef]
- Ryan, A.M.; Sullivan, E.S. Impact of Musculoskeletal Degradation on Cancer Outcomes and Strategies for Management in Clinical Practice. Proc. Nutr. Soc. 2021, 80, 73–91. [Google Scholar] [CrossRef]
- Sarhill, N.; Mahmoud, F.; Walsh, D.; Nelson, K.A.; Komurcu, S.; Davis, M.; LeGrand, S.; Abdullah, O.; Rybicki, L. Evaluation of Nutritional Status in Advanced Metastatic Cancer. Support. Care Cancer 2003, 11, 652–659. [Google Scholar] [CrossRef]
- Caccialanza, R.; Cereda, E.; Pinto, C.; Cotogni, P.; Farina, G.; Gavazzi, C.; Gandini, C.; Nardi, M.; Zagonel, V.; Pedrazzoli, P. Awareness and Consideration of Malnutrition among Oncologists: Insights from an Exploratory Survey. Nutrition 2016, 32, 1028–1032. [Google Scholar] [CrossRef]
- Durán-Poveda, M.; Jimenez-Fonseca, P.; Sirvent-Ochando, M.; García-Luna, P.P.; Pereira-Cunill, J.L.; Lema-Marqués, B.; Parejo-Arrondo, M.T.; Belda-Iniesta, C. Integral Nutritional Approach to the Care of Cancer Patients: Results from a Delphi Panel. Clin. Transl. Oncol. 2018, 20, 1202–1211. [Google Scholar] [CrossRef]
- Spiro, A.; Baldwin, C.; Patterson, A.; Thomas, J.; Andreyev, H.J.N. The Views and Practice of Oncologists towards Nutritional Support in Patients Receiving Chemotherapy. Br. J. Cancer 2006, 95, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, H.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Criteria for the Diagnosis of Malnutrition—A Consensus Report from the Global Clinical Nutrition Community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martin, L.; Gioulbasanis, I.; Senesse, P.; Baracos, V.E. Cancer-Associated Malnutrition and CT-Defined Sarcopenia and Myosteatosis Are Endemic in Overweight and Obese Patients. JPEN J. Parenter. Enter. Nutr. 2020, 44, 227–238. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-Associated Cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Ruan, X.; Nakyeyune, R.; Shao, Y.; Shen, Y.; Niu, C.; Zang, Z.; Miles, T.; Liu, F. Nutritional Screening Tools for Adult Cancer Patients: A Hierarchical Bayesian Latent-Class Meta-Analysis. Clin. Nutr. 2020, S0261-5614(20)30505-7. [Google Scholar] [CrossRef]
- Blauwhoff-Buskermolen, S.; Versteeg, K.S.; de van der Schueren, M.A.E.; den Braver, N.R.; Berkhof, J.; Langius, J.A.E.; Verheul, H.M.W. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2016, 34, 1339–1344. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Sawyer, M.B.; Wischmeyer, P.E.; Baracos, V.E. Nutrition Modulation of Gastrointestinal Toxicity Related to Cancer Chemotherapy: From Preclinical Findings to Clinical Strategy. JPEN J. Parenter. Enter. Nutr. 2011, 35, 74–90. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A Practical and Precise Approach to Quantification of Body Composition in Cancer Patients Using Computed Tomography Images Acquired during Routine Care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and Clinical Implications of Sarcopenic Obesity in Patients with Solid Tumours of the Respiratory and Gastrointestinal Tracts: A Population-Based Study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Greenlee, H.; Unger, J.M.; LeBlanc, M.; Ramsey, S.; Hershman, D.L. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol. Biomark. Prev. 2017, 26, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Berardi, G.; Antonelli, G.; Colasanti, M.; Meniconi, R.; Guglielmo, N.; Laurenzi, A.; Ferretti, S.; Levi Sandri, G.B.; Spagnoli, A.; Moschetta, G.; et al. Association of Sarcopenia and Body Composition with Short-Term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg. 2020, 155, e203336. [Google Scholar] [CrossRef]
- Palle, S.S.; Møllehave, L.T.; Taheri-Kadkhoda, Z.; Johansen, S.; Larsen, L.; Hansen, J.W.; Jensen, N.K.G.; Elingaard, A.O.; Møller, A.H.; Larsen, K.; et al. Multi-Frequency Bioelectrical Impedance Analysis (BIA) Compared to Magnetic Resonance Imaging (MRI) for Estimation of Fat-Free Mass in Colorectal Cancer Patients Treated with Chemotherapy. Clin. Nutr. ESPEN 2016, 16, 8–15. [Google Scholar] [CrossRef]
- Casirati, A.; Vandoni, G.; Della Valle, S.; Greco, G.; Platania, M.; Colatruglio, S.; Lalli, L.; Gavazzi, C. Nutritional Status and Body Composition Assessment in Patients with a New Diagnosis of Advanced Solid Tumour: Exploratory Comparison of Computed Tomography and Bioelectrical Impedance Analysis. Clin. Nutr. 2021, 40, 1268–1273. [Google Scholar] [CrossRef]
- Qin, L.; Tian, Q.; Zhu, W.; Wu, B. The Validity of the GLIM Criteria for Malnutrition in Hospitalized Patients with Gastric Cancer. Nutr. Cancer 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yavuzsen, T.; Davis, M.P.; Walsh, D.; LeGrand, S.; Lagman, R. Systematic Review of the Treatment of Cancer-Associated Anorexia and Weight Loss. J. Clin. Oncol. 2005, 23, 8500–8511. [Google Scholar] [CrossRef] [PubMed]
- Aprile, G.; Ramoni, M.; Keefe, D.; Sonis, S. Application of Distance Matrices to Define Associations between Acute Toxicities in Colorectal Cancer Patients Receiving Chemotherapy. Cancer 2008, 112, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, E.; Buoro, V.; Cinausero, M.; Caccialanza, R.; Turri, A.; Fanotto, V.; Basile, D.; Vitale, M.G.; Ermacora, P.; Cardellino, G.G.; et al. Sarcopenia in Gastric Cancer: When the Loss Costs Too Much. Gastric Cancer 2017, 20, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Álvaro Sanz, E.; Abilés, J.; Garrido Siles, M.; Pérez Ruíz, E.; Alcaide García, J.; Rueda Domínguez, A. Impact of Weight Loss on Cancer Patients’ Quality of Life at the Beginning of the Chemotherapy. Support. Care Cancer 2021, 29, 627–634. [Google Scholar] [CrossRef]
- Lin, T.; Yang, J.; Hong, X.; Yang, Z.; Ge, T.; Wang, M. Nutritional Status in Patients with Advanced Lung Cancer Undergoing Chemotherapy: A Prospective Observational Study. Nutr. Cancer 2020, 72, 1225–1230. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. An Update on Pharmacotherapies in Active Development for the Management of Cancer Regimen-Associated Oral Mucositis. Expert Opin. Pharmacother. 2020, 21, 541–548. [Google Scholar] [CrossRef]
- Neoh, M.K.; Abu Zaid, Z.; Mat Daud, Z.A.; Md Yusop, N.B.; Ibrahim, Z.; Abdul Rahman, Z.; Jamhuri, N. Changes in Nutrition Impact Symptoms, Nutritional and Functional Status during Head and Neck Cancer Treatment. Nutrients 2020, 12, 1225. [Google Scholar] [CrossRef]
- Lodewijckx, E.; Kenis, C.; Flamaing, J.; Debruyne, P.; De Groof, I.; Focan, C.; Cornélis, F.; Verschaeve, V.; Bachmann, C.; Bron, D.; et al. Unplanned Hospitalizations in Older Patients with Cancer: Occurrence and Predictive Factors. J. Geriatr. Oncol. 2020, 12, 368–374. [Google Scholar] [CrossRef]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Pugnaloni, S.; Alia, S.; Mancini, M.; Santoro, V.; Di Paolo, A.; Rabini, R.A.; Fiorini, R.; Sabbatinelli, J.; Fabri, M.; Mazzanti, L.; et al. A Study on the Relationship between Type 2 Diabetes and Taste Function in Patients with Good Glycemic Control. Nutrients 2020, 12, 1112. [Google Scholar] [CrossRef]
- De Vries, Y.C.; Boesveldt, S.; Kelfkens, C.S.; Posthuma, E.E.; Berg, M.M.G.A.v.D.; de Kruif, J.T.C.M.; Haringhuizen, A.; Sommeijer, D.W.; Buist, N.; Grosfeld, S.; et al. Taste and Smell Perception and Quality of Life during and after Systemic Therapy for Breast Cancer. Breast Cancer Res. Treat. 2018, 170, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, R.; Goda, M.; Fujimoto, C.; Kanno, K.; Nobe, M.; Kitamura, Y.; Abe, K.; Kawai, M.; Matsumoto, H.; Sakai, T.; et al. Effects of Chemotherapy on Gene Expression of Lingual Taste Receptors in Patients with Head and Neck Cancer. Laryngoscope 2016, 126, E103–E109. [Google Scholar] [CrossRef]
- Tuca, A.; Jimenez-Fonseca, P.; Gascón, P. Clinical Evaluation and Optimal Management of Cancer Cachexia. Crit. Rev. Oncol. Hematol. 2013, 88, 625–636. [Google Scholar] [CrossRef]
- Argilés, J.M.; López-Soriano, F.J.; Busquets, S. Mechanisms and Treatment of Cancer Cachexia. Nutr. Metab. Cardiovasc. Dis. 2013, 23 (Suppl. 1), S19–S24. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Molfino, A.; Lucia, S.; Rossi Fanelli, F. Cachexia: A Preventable Comorbidity of Cancer. A T.A.R.G.E.T. Approach. Crit. Rev. Oncol. Hematol. 2015, 94, 251–259. [Google Scholar] [CrossRef]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer Cachexia Prevention via Physical Exercise: Molecular Mechanisms. J. Cachexia Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef]
- Ukovic, B.; Porter, J. Nutrition Interventions to Improve the Appetite of Adults Undergoing Cancer Treatment: A Systematic Review. Support. Care Cancer 2020, 28, 4575–4583. [Google Scholar] [CrossRef]
- Braud, A.; Boucher, Y. Taste Disorder’s Management: A Systematic Review. Clin. Oral Investig. 2020, 24, 1889–1908. [Google Scholar] [CrossRef]
- Caccialanza, R.; Cereda, E.; Klersy, C.; Brugnatelli, S.; Borioli, V.; Ferrari, A.; Caraccia, M.; Lobascio, F.; Pagani, A.; Delfanti, S.; et al. Early Intravenous Administration of Nutritional Support (IVANS) in Metastatic Gastric Cancer Patients at Nutritional Risk, Undergoing First-Line Chemotherapy: Study Protocol of a Pragmatic, Randomized, Multicenter, Clinical Trial. Ther. Adv. Med. Oncol. 2020, 12, 1758835919890281. [Google Scholar] [CrossRef]
- Baldwin, C.; Spiro, A.; Ahern, R.; Emery, P.W. Oral Nutritional Interventions in Malnourished Patients with Cancer: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2012, 104, 371–385. [Google Scholar] [CrossRef] [Green Version]
- Bourdel-Marchasson, I.; Blanc-Bisson, C.; Doussau, A.; Germain, C.; Blanc, J.-F.; Dauba, J.; Lahmar, C.; Terrebonne, E.; Lecaille, C.; Ceccaldi, J.; et al. Nutritional Advice in Older Patients at Risk of Malnutrition during Treatment for Chemotherapy: A Two-Year Randomized Controlled Trial. PLoS ONE 2014, 9, e108687. [Google Scholar] [CrossRef]
- Paccagnella, A.; Morello, M.; Da Mosto, M.C.; Baruffi, C.; Marcon, M.L.; Gava, A.; Baggio, V.; Lamon, S.; Babare, R.; Rosti, G.; et al. Early Nutritional Intervention Improves Treatment Tolerance and Outcomes in Head and Neck Cancer Patients Undergoing Concurrent Chemoradiotherapy. Support. Care Cancer 2010, 18, 837–845. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Aapro, M. From Guidelines to Clinical Practice: A Roadmap for Oncologists for Nutrition Therapy for Cancer Patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919880084. [Google Scholar] [CrossRef] [Green Version]
- Caccialanza, R.; Cereda, E.; De Lorenzo, F.; Farina, G.; Pedrazzoli, P.; AIOM-SINPE-FAVO Working Group. To Fast, or Not to Fast before Chemotherapy, That Is the Question. BMC Cancer 2018, 18, 337. [Google Scholar] [CrossRef] [Green Version]
- Caccialanza, R.; Aprile, G.; Cereda, E.; Pedrazzoli, P. Fasting in Oncology: A Word of Caution. Nat. Rev. Cancer 2019, 19, 177. [Google Scholar] [CrossRef] [Green Version]
- Sadeghian, M.; Rahmani, S.; Khalesi, S.; Hejazi, E. A Review of Fasting Effects on the Response of Cancer to Chemotherapy. Clin. Nutr. 2020, S0261-5614(20)30580. [Google Scholar] [CrossRef]
- Pistollato, F.; Forbes-Hernandez, T.Y.; Iglesias, R.C.; Ruiz, R.; Zabaleta, M.E.; Dominguez, I.; Cianciosi, D.; Quiles, J.L.; Giampieri, F.; Battino, M. Effects of Caloric Restriction on Immunosurveillance, Microbiota and Cancer Cell Phenotype: Possible Implications for Cancer Treatment. Semin. Cancer Biol. 2020, S1044-579X(20)30255-8. [Google Scholar] [CrossRef]
- Álvaro Sanz, E.; Abilés, J.; Garrido Siles, M.; Rivas Ruíz, F.; Tortajada Goitia, B.; Domínguez, A.R. Evaluation of a Protocol to Detect Malnutrition and Provide Nutritional Care for Cancer Patients Undergoing Chemotherapy. Sci. Rep. 2020, 10, 21186. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Murphy, E.A.; Carson, J.A. The Impact of Immune Cells on the Skeletal Muscle Microenvironment during Cancer Cachexia. Front. Physiol. 2020, 11, 1037. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.P.; Li, Y.; Ghosh, S.; Sass, S.; Smylie, M.; Walker, J.; Sawyer, M.B. Body Composition Is Prognostic and Predictive of Ipilimumab Activity in Metastatic Melanoma. J. Cachexia Sarcopenia Muscle 2020, 11, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Roch, B.; Coffy, A.; Jean-Baptiste, S.; Palaysi, E.; Daures, J.-P.; Pujol, J.-L.; Bommart, S. Cachexia—Sarcopenia as a Determinant of Disease Control Rate and Survival in Non-Small Lung Cancer Patients Receiving Immune-Checkpoint Inhibitors. Lung Cancer 2020, 143, 19–26. [Google Scholar] [CrossRef]
- Floris, G.; Richard, F.; Hamy, A.-S.; Jongen, L.; Wildiers, H.; Ardui, J.; Punie, K.; Smeets, A.; Berteloot, P.; Vergote, I.; et al. Body Mass Index and Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2021, 113, 146–153. [Google Scholar] [CrossRef]
- Matsubara, T.; Takamori, S.; Haratake, N.; Toyozawa, R.; Miura, N.; Shimokawa, M.; Yamaguchi, M.; Seto, T.; Takenoyama, M. The Impact of Immune-Inflammation-Nutritional Parameters on the Prognosis of Non-Small Cell Lung Cancer Patients Treated with Atezolizumab. J. Thorac. Dis. 2020, 12, 1520–1528. [Google Scholar] [CrossRef]
- Wang, J.-B.; Li, P.; Liu, X.-L.; Zheng, Q.-L.; Ma, Y.-B.; Zhao, Y.-J.; Xie, J.-W.; Lin, J.-X.; Lu, J.; Chen, Q.-Y.; et al. An Immune Checkpoint Score System for Prognostic Evaluation and Adjuvant Chemotherapy Selection in Gastric Cancer. Nat. Commun. 2020, 11, 6352. [Google Scholar] [CrossRef]
- Jiang, Y.; Tu, X.; Zhang, X.; Liao, H.; Han, S.; Jiang, W.; Zheng, Y.; Zhao, P.; Tong, Z.; Fu, Q.; et al. Nutrition and Metabolism Status Alteration in Advanced Hepatocellular Carcinoma Patients Treated with Anti-PD-1 Immunotherapy. Support. Care Cancer 2020, 28, 5569–5579. [Google Scholar] [CrossRef]
- Johannet, P.; Sawyers, A.; Qian, Y.; Kozloff, S.; Gulati, N.; Donnelly, D.; Zhong, J.; Osman, I. Baseline Prognostic Nutritional Index and Changes in Pretreatment Body Mass Index Associate with Immunotherapy Response in Patients with Advanced Cancer. J. Immunother. Cancer 2020, 8, e001674. [Google Scholar] [CrossRef]
- Shoji, F.; Takeoka, H.; Kozuma, Y.; Toyokawa, G.; Yamazaki, K.; Ichiki, M.; Takeo, S. Pretreatment Prognostic Nutritional Index as a Novel Biomarker in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Lung Cancer 2019, 136, 45–51. [Google Scholar] [CrossRef]
- Fujii, H.; Makiyama, A.; Iihara, H.; Okumura, N.; Yamamoto, S.; Imai, T.; Arakawa, S.; Kobayashi, R.; Tanaka, Y.; Yoshida, K.; et al. Cancer Cachexia Reduces the Efficacy of Nivolumab Treatment in Patients With Advanced Gastric Cancer. Anticancer Res. 2020, 40, 7067–7075. [Google Scholar] [CrossRef]
- Akce, M.; Liu, Y.; Zakka, K.; Martini, D.J.; Draper, A.; Alese, O.B.; Shaib, W.L.; Wu, C.; Wedd, J.P.; Sellers, M.T.; et al. Impact of Sarcopenia, BMI, and Inflammatory Biomarkers on Survival in Advanced Hepatocellular Carcinoma Treated With Anti-PD-1 Antibody. Am. J. Clin. Oncol. 2021, 44, 74–81. [Google Scholar] [CrossRef]
- Nishioka, N.; Naito, T.; Notsu, A.; Mori, K.; Kodama, H.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kobayashi, H.; Omori, S.; et al. Unfavorable Impact of Decreased Muscle Quality on the Efficacy of Immunotherapy for Advanced Non-small Cell Lung Cancer. Cancer Med. 2020, 10, 247–256. [Google Scholar] [CrossRef]
- Guzman-Prado, Y.; Ben Shimol, J.; Samson, O. Body Mass Index and Immune-Related Adverse Events in Patients on Immune Checkpoint Inhibitor Therapies: A Systematic Review and Meta-Analysis. Cancer Immunol. Immunother. 2021, 70, 89–100. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another Side of the Association between Body Mass Index (BMI) and Clinical Outcomes of Cancer Patients Receiving Programmed Cell Death Protein-1 (PD-1)/ Programmed Cell Death-Ligand 1 (PD-L1) Checkpoint Inhibitors: A Multicentre Analysis of Immune-Related Adverse Events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [CrossRef]
- Cortellini, A.; Ricciuti, B.; Tiseo, M.; Bria, E.; Banna, G.L.; Aerts, J.G.; Barbieri, F.; Giusti, R.; Cortinovis, D.L.; Migliorino, M.R.; et al. Baseline BMI and BMI Variation during First Line Pembrolizumab in NSCLC Patients with a PD-L1 Expression ≥ 50%: A Multicenter Study with External Validation. J. Immunother. Cancer 2020, 8, e001403. [Google Scholar] [CrossRef]
- Martini, D.J.; Kline, M.R.; Liu, Y.; Shabto, J.M.; Williams, M.A.; Khan, A.I.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.T.; et al. Adiposity May Predict Survival in Patients with Advanced Stage Cancer Treated with Immunotherapy in Phase 1 Clinical Trials. Cancer 2020, 126, 575–582. [Google Scholar] [CrossRef]
- Grignol, V.P.; Smith, A.D.; Shlapak, D.; Zhang, X.; Del Campo, S.M.; Carson, W.E. Increased Visceral to Subcutaneous Fat Ratio Is Associated with Decreased Overall Survival in Patients with Metastatic Melanoma Receiving Anti-Angiogenic Therapy. Surg. Oncol. 2015, 24, 353–358. [Google Scholar] [CrossRef]
- Turner, D.C.; Kondic, A.G.; Anderson, K.M.; Robinson, A.G.; Garon, E.B.; Riess, J.W.; Jain, L.; Mayawala, K.; Kang, J.; Ebbinghaus, S.W.; et al. Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin. Cancer Res. 2018, 24, 5841–5849. [Google Scholar] [CrossRef] [Green Version]
- Cotogni, P.; Ossola, M.; Passera, R.; Monge, T.; Fadda, M.; De Francesco, A.; Bozzetti, F. Home Parenteral Nutrition versus Artificial Hydration in Malnourished Patients with Cancer in Palliative Care: A Prospective, Cohort Survival Study. BMJ Support. Palliat. Care 2020, 1–7. [Google Scholar] [CrossRef]
- Baumstarck, K.; Boyer, L.; Pauly, V.; Orleans, V.; Marin, A.; Fond, G.; Morin, L.; Auquier, P.; Salas, S. Use of Artificial Nutrition near the End of Life: Results from a French National Population-Based Study of Hospitalized Cancer Patients. Cancer Med. 2020, 9, 530–540. [Google Scholar] [CrossRef]
- Blum, D.; Jensen, W.; Ullrich, A.; Hlawatsch, C.; Bokemeyer, C.; Oechsle, K. Tipping Point: When Patients Stop Eating and Drinking in the Last Phase of Their Life. Clin. Nutr. ESPEN 2020, 38, 280–282. [Google Scholar] [CrossRef]
- Albanesi, B.; Piredda, M.; Marchetti, A.; Mastroianni, C.; Magnani, C.; Artico, M.; D’Angelo, D.; Lusignani, M.; Ianni, A.; De Marinis, M.G. Oncology and Palliative Care Nurses’ Knowledge and Attitudes Toward Artificial Nutrition and Hydration for Patients at End of Life in Italy: A Cross-Sectional Survey. Cancer Nurs. 2021, 44, E99–E107. [Google Scholar] [CrossRef]
- Caccialanza, R.; Goldwasser, F.; Marschal, O.; Ottery, F.; Schiefke, I.; Tilleul, P.; Zalcman, G.; Pedrazzoli, P. Unmet Needs in Clinical Nutrition in Oncology: A Multinational Analysis of Real-World Evidence. Ther. Adv. Med. Oncol. 2020, 12, 1758835919899852. [Google Scholar] [CrossRef]
- Rossi, R.; Serra, P.; Suzzi, M.; Guerra, D.; Bilotta, S.; Ricci, M.; Pallotti, M.C.; Ibrahim, T.; Frassineti, G.L.; Zavoiu, V.; et al. The Challenge for Nutritional Care in a Cancer Center: The Need for Integration between Clinical Nutritionist, Oncologist, and Palliative Care Physician. Curr. Probl. Cancer 2020, 44, 100618. [Google Scholar] [CrossRef]
- Sharour, L.A. Improving Oncology Nurses’ Knowledge, Self-Confidence, and Self-Efficacy in Nutritional Assessment and Counseling for Patients with Cancer: A Quasi-Experimental Design. Nutrition 2019, 62, 131–134. [Google Scholar] [CrossRef]
- Murphy, J.L.; Munir, F.; Davey, F.; Miller, L.; Cutress, R.; White, R.; Lloyd, M.; Roe, J.; Granger, C.; Burden, S.; et al. The Provision of Nutritional Advice and Care for Cancer Patients: A UK National Survey of Healthcare Professionals. Support. Care Cancer 2021, 29, 2435–2442. [Google Scholar] [CrossRef]
- Paulsen, M.M.; Paur, I.; Gjestland, J.; Henriksen, C.; Varsi, C.; Tangvik, R.J.; Andersen, L.F. Effects of Using the MyFood Decision Support System on Hospitalized Patients’ Nutritional Status and Treatment: A Randomized Controlled Trial. Clin. Nutr. 2020, 39, 3607–3617. [Google Scholar] [CrossRef]
- NUTRIENT—App su Google Play. Available online: https://play.google.com/store/apps/details?id=com.progettidiimpresa.nutrient.it.android&hl=it&gl=US (accessed on 23 March 2021).
- Groß, S.E.; Weidner, D.; Cecon, N.; Pfaff, H.; Strauch, C.; Scholten, N. Does Basic Information Concerning Nutrition Improve the Information Needs of Breast Cancer Patients? An Evaluation. Support. Care Cancer 2020, 28, 5419–5427. [Google Scholar] [CrossRef] [Green Version]
- Wolf, P.G.; Manero, J.; Harold, K.B.; Chojnacki, M.; Kaczmarek, J.; Liguori, C.; Arthur, A. Educational Video Intervention Improves Knowledge and Self-Efficacy in Identifying Malnutrition among Healthcare Providers in a Cancer Center: A Pilot Study. Support. Care Cancer 2020, 28, 683–689. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprile, G.; Basile, D.; Giaretta, R.; Schiavo, G.; La Verde, N.; Corradi, E.; Monge, T.; Agustoni, F.; Stragliotto, S. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients 2021, 13, 1196. https://doi.org/10.3390/nu13041196
Aprile G, Basile D, Giaretta R, Schiavo G, La Verde N, Corradi E, Monge T, Agustoni F, Stragliotto S. The Clinical Value of Nutritional Care before and during Active Cancer Treatment. Nutrients. 2021; 13(4):1196. https://doi.org/10.3390/nu13041196
Chicago/Turabian StyleAprile, Giuseppe, Debora Basile, Renato Giaretta, Gessica Schiavo, Nicla La Verde, Ettore Corradi, Taira Monge, Francesco Agustoni, and Silvia Stragliotto. 2021. "The Clinical Value of Nutritional Care before and during Active Cancer Treatment" Nutrients 13, no. 4: 1196. https://doi.org/10.3390/nu13041196