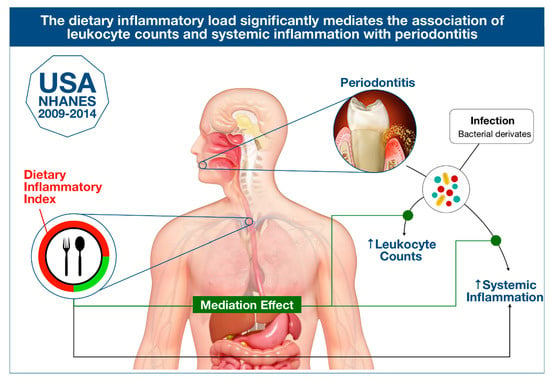
Association between Dietary Inflammatory Index and Periodontitis: A Cross-Sectional and Mediation Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Diet. and Dietary Inflammatory Index
2.3. Periodontal Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. DII, PD and Systemic Inflammation
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bates, C.J.; Hamer, M.; Mishra, G.D. Redox-Modulatory Vitamins and Minerals That Prospectively Predict Mortality in Older British People: The National Diet and Nutrition Survey of People Aged 65 Years and Over. Br. J. Nutr. 2011, 105, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Aucott, L.S.; McNeill, G. Nutritional Status and Subsequent All-Cause Mortality in Men and Women Aged 75 Years or over Living in the Community. Br. J. Nutr. 2007, 98, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.; Guralnik, J.; Fried, L.P. Serum Antioxidants, Inflammation, and Total Mortality in Older Women. Am. J. Epidemiol. 2006, 163, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Calvo, N.; Martínez-Gonzàlez, M.Á. Vitamin C Intake Is Inversely Associated with Cardiovascular Mortality in a Cohort of Spanish Graduates: The SUN Project. Nutrients 2017, 9, 954. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Arellano, A.; Ramallal, R.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Shivappa, N.; Schröder, H.; Hébert, J.R.; Ros, E.; Gómez-Garcia, E.; et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the PREDIMED Study. Nutrients 2015, 7, 4124–4138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Wang, K.; Sun, J.Z.; Wu, Q.X.; Li, Z.Y.; Li, D.X.; Xiong, Y.F.; Zhong, G.C.; Shi, Y.; Li, Q.; Zheng, J.; et al. Long-Term Anti-Inflammatory Diet in Relation to Improved Breast Cancer Prognosis: A Prospective Cohort Study. NPJ Breast Cancer 2020, 6, 36. [Google Scholar] [CrossRef]
- Vadell, A.K.E.; Bärebring, L.; Hulander, E.; Gjertsson, I.; Lindqvist, H.M.; Winkvist, A. Anti-Inflammatory Diet In Rheumatoid Arthritis (ADIRA)—a Randomized, Controlled Crossover Trial Indicating Dieffects on Disease Activity. Am. J. Clin. Nutr. 2020, 111, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Woelber, J.P.; Gärtner, M.; Breuninger, L.; Anderson, A.; König, D.; Hellwig, E.; Al-Ahmad, A.; Vach, K.; Dötsch, A.; Ratka-Krüger, P.; et al. The Influence of an Anti-Inflammatory Diet on Gingivitis. A Randomized Controlled Trial. J. Clin. Periodontol. 2019, 46, 481–490. [Google Scholar] [CrossRef]
- Watanabe, K. Anti-Inflammatory Diet: Necessity of Scientific Spotlight and Challenges. Complement. Ther. Med. 2020, 50, 2019–2020. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Subar, A.F.; Reedy, J. Examining Dietary Patterns in Relation to Chronic Disease: Matching Measures and Methods to Questions of Interest. Circulation 2015, 132, 790–793. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef] [Green Version]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A New Dietary Inflammatory Index Predicts Interval Changes in Serum High-Sensitivity. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Carson, S.J.; Burns, J. Impact of Smoking on Tooth Loss in Adults. Evid. Based Dent. 2016, 17, 73–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, B.; Newman, J.D.; Woolf, K.; Ganguzza, L.; Guo, Y.; Allen, N.; Zhong, J.; Fisher, E.A.; Slater, J. Anti-Inflammatory Effects of a Vegan Diet Versus the American Heart Association-Recommended Diet in Coronary Artery Disease Trial. J. Am. Heart Assoc. 2018, 7, e011367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between Dietary Inflammatory Index and Inflammatory Markers in the HELENA Study. Mol. Nutr. Food Res. 2017, 61, 1–23. [Google Scholar] [CrossRef]
- Ramallal, R.; Toledo, E.; Martínez, J.A.; Shivappa, N.; Hébert, J.R.; Martínez-González, M.A.; Ruiz-Canela, M. Inflammatory Potential of Diet, Weight Gain, and Incidence of Overweight/Obesity: The SUN Cohort. Obesity 2017, 25, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Neufcourt, L.; Assmann, K.E.; Fezeu, L.K.; Touvier, M.; Graffouillère, L.; Shivappa, N.; Hébert, J.R.; Wirth, M.D.; Hercberg, S.; Galan, P.; et al. Prospective Association between the Dietary Inflammatory Index and Metabolic Syndrome: Findings from the SU.VI.MAX Study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Chrepa, V.; Shivappa, N.; Wirth, M.; Hébert, J.; Koyanagi, A.; Tyrovolas, S. Diet-Borne Systemic Inflammation Is Associated with Prevalent Tooth Loss. Clin. Nutr. 2018, 37, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: A Call for Global Action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990–2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Helal, O.; Göstemeyer, G.; Krois, J.; Sayed, K.F.E.; Graetz, C.; Schwendicke, F. Predictors for Tooth Loss in Periodontitis Patients: Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- De Pablo, P.; Chapple, I.L.C.; Buckley, C.D.; Dietrich, T. Periodontitis in Systemic Rheumatic Diseases. Nat. Rev. Rheumatol. 2009, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Buset, S.L.; Walter, C.; Friedmann, A.; Weiger, R.; Borgnakke, W.S.; Zitzmann, N.U. Are Periodontal Diseases Really Silent? A Systematic Review of Their Effect on Quality of Life. J. Clin. Periodontol. 2016, 43, 333–344. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Bellini, D.H.; Chambrone, L.; Alcoforado, G.; Mendes, J.J. The Impact of Nonsurgical Periodontal Treatment on Oral Health-Related Quality of Life: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2020, 24, 585–596. [Google Scholar] [CrossRef]
- Kondo, K.; Ishikado, A.; Morino, K.; Nishio, Y.; Ugi, S.; Kajiwara, S.; Kurihara, M.; Iwakawa, H.; Nakao, K.; Uesaki, S.; et al. A High-Fiber, Low-Fat Diet Improves Periodontal Disease Markers in High-Risk Subjects: A Pilot Study. Nutr. Res. 2014, 34, 491–498. [Google Scholar] [CrossRef]
- Nascimento, G.G.; Peres, M.A.; Mittinty, M.N.; Peres, K.G.; Do, L.G.; Horta, B.L.; Gigante, D.P.; Corrêa, M.B.; Demarco, F.F. Diet-Induced Overweight and Obesity and Periodontitis Risk: An Application of the Parametric g-Formula in the 1982 Pelotas Birth Cohort. Am. J. Epidemiol. 2017, 185, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Raindi, D. Nutrition and Periodontal Disease. Dent. Update 2016, 43, 66–72. [Google Scholar] [CrossRef]
- Alhassani, A.A.; Hu, F.B.; Rimm, E.B.; Li, Y.; Rosner, B.A.; Willett, W.C.; Joshipura, K.J. Dietary Flavonoid Intake and Risk of Periodontitis. J. Periodontol. 2020, 91, 1057–1066. [Google Scholar] [CrossRef]
- Lachat, C.; Hawwash, D.; Ocké, M.C.; Berg, C.; Forsum, E.; Hörnell, A.; Larsson, C.; Sonestedt, E.; Wirfält, E.; Åkesson, A.; et al. Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-Nut): An Extension of the STROBE Statement. PLoS Med. 2016, 13, e1002036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A. Accuracy of NHANES Periodontal Examination Protocols. J. Dent. Res. 2010, 89, 1208–1213. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Kaye, E.K. Nutrition, Dietary Guidelines and Optimal Periodontal Health. Periodontology 2000 2012, 58, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Laiola, M.; De Filippis, F.; Vitaglione, P.; Ercolini, D. A Mediterranean Diet Intervention Reduces the Levels of Salivary Periodontopathogenic Bacteria in Overweight and Obese Subjects. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Bullon, P.; Morillo, J.M.; Battino, M.; Quiles, J.L.; Ramirez-Tortosa, M.C. The Relationship between Insulin Resistance and Periodontitis Is Not Affected by Mediterranean Diet in a Spanish Population. Arch. Oral Biol. 2017, 77, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Slawik, S.; Staufenbiel, I.; Schilke, R.; Nicksch, S.; Weinspach, K.; Stiesch, M.; Eberhard, J. Probiotics Affect the Clinical Inflammatory Parameters of Experimental Gingivitis in Humans. Eur. J. Clin. Nutr. 2011, 65, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G. Probiotics and Periodontal Health. J. Med. Life 2011, 4, 387. [Google Scholar]
- Chatterjee, A.; Bhattacharya, H.; Kandwal, A. Probiotics in Periodontal Health and Disease. J. Indian Soc. Periodontol. 2011, 15, 23. [Google Scholar] [CrossRef]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a Vegan Diet on the Human Salivary Microbiota. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Tomofuji, T.; Ekuni, D.; Irie, K.; Azuma, T.; Endo, Y.; Tamaki, N.; Sanbe, T.; Murakami, J.; Yamamoto, T.; Morita, M. Preventive Effects of a Cocoa-Enriched Diet on Gingival Oxidative Stress in Experimental Periodontitis. J. Periodontol. 2009, 80, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, S.; Imfeld, T.; Schicht, O.; Rath, C.; Persson, R.E.; Persson, G.R. The Impact of the Stone Age Diet on Gingival Conditions in the Absence of Oral Hygiene. J. Periodontol. 2009, 80, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Bremer, K.; Vach, K.; König, D.; Hellwig, E.; Ratka-Krüger, P.; Al-Ahmad, A.; Tennert, C. An Oral Health Optimized Diet Can Reduce Gingival and Periodontal Inflammation in Humans—A Randomized Controlled Pilot Study. BMC Oral Health 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, D.; Ceriello, A.; Esposito, K. The Effects of Diet on Inflammation Emphasis on the Metabolic Syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Rhodes, B.; Fürnrohr, B.G.; Vyse, T.J. C-Reactive Protein in Rheumatology: Biology and Genetics. Nat. Rev. Rheumatol. 2011, 7, 282–289. [Google Scholar] [CrossRef]
- Craig, R.G.; Yip, J.K.; So, M.K.; Boylan, R.J.; Socransky, S.S.; Haffajee, A.D. Relationship of Destructive Periodontal Disease to the Acute-Phase Response. J. Periodontol. 2003, 74, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J. Dynamic Interactions of Neutrophils and Biofilms. J. Oral Microbiol. 2014, 6, 1–10. [Google Scholar] [CrossRef]
- Hajishengallis, G. New Developments in Neutrophil Biology and Periodontitis. Periodontology 2000 2020, 82, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Ebersole, J.L.; Dawson, D.R.; Morford, L.A.; Peyyala, R.; Miller, C.S.; Gonzaléz, O.A. Periodontal Disease Immunology: “Double Indemnity” in Protecting the Host. Periodontology 2000 2013, 62, 163–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gkranias, N.; Bhowruth, D.; Khan, T.; Orlandi, M.; Suvan, J.; Masi, S.; Tsakos, G.; Hurel, S.; Hingorani, A.D.; et al. Systemic Effects of Periodontitis Treatment in Patients with Type 2 Diabetes: A 12 Month, Single-Centre, Investigator-Masked, Randomised Trial. Lancet Diabetes Endocrinol. 2018, 6, 954–965. [Google Scholar] [CrossRef]
- Deng, F.E.; Shivappa, N.; Tang, Y.; Mann, J.R.; Hebert, J.R. Association between Diet-Related Inflammation, All-Cause, All-Cancer, and Cardiovascular Disease Mortality, with Special Focus on Prediabetics: Findings from NHANES III. Eur. J. Nutr. 2017, 56, 1085–1093. [Google Scholar] [CrossRef]
- Machado, V.; Botelho, J.; Mascarenhas, P.; Cavacas, M.A.; Alves, R.; Mendes, J.J. Partial Recording Protocols Performance on the Assessment of Periodontitis Severity and Extent: Bias Magnitudes, Sensibility, and Specificity. Rev. Port. Estomatol. Med. Dent. E Cir. Maxilofac. 2018, 59, 145–153. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Mendes, J.J. The 2018 Periodontitis Case Definition Improves Accuracy Performance of Full-Mouth Partial Diagnostic Protocols. Sci. Rep. 2020, 10, 7093. [Google Scholar] [CrossRef]

| NHANES 2009–2010 (n = 3238) | NHANES 2011–2012 (n = 3323) | NHANES 2013–2014 (n = 3617) | NHANES 2009–2014 (n = 10,178) | |
|---|---|---|---|---|
| Age (years), mean (SD) | 52.2 (14.4) | 51.8 (14.2) | 52.0 (14.3) | 52.0 (14.3) |
| Gender, n (%) | ||||
| Males | 1623 (50.1) | 1643 (49.4) | 1752 (48.4) | 5018 (49.3) |
| Females | 1615 (49.9) | 1680 (50.6) | 1865 (51.6) | 5160 (50.7) |
| Race/ethnicity, n (%) | ||||
| Mexican American | 585 (18.1) | 355 (10.7) | 491 (13.6) | 1431 (14.1) |
| Non-Hispanic White | 339 (10.5) | 1226 (36.9) | 1562 (43.2) | 3127 (30.7) |
| Non-Hispanic Black | 1555 (48.0) | 839 (25.2) | 704 (19.5) | 3098 (30.4) |
| Other Hispanic | 586 (18.1) | 343 (10.3) | 322 (8.9) | 1251 (12.3) |
| Other race | 173 (5.3) | 560 (16.9) | 538 (14.9) | 1271 (12.5) |
| Education level, n (%) | ||||
| <High school | 401 (12.4) | 318 (9.6) | 283 (7.9) | 1000 (9.8) |
| High school | 1203 (37.2) | 1135 (34.2) | 1217 (33.7) | 3555 (34.9) |
| >High school | 1634 (50.5) | 1870 (56.2) | 2117 (58.5) | 5623 (55.2) |
| Smoking status, n (%) | ||||
| Never | 1777 (54.9) | 1900 (57.2) | 2046 (56.6) | 5723 (56.2) |
| Former | 841 (26.0) | 813 (24.5) | 905 (25.0) | 2559 (25.1) |
| Current | 620 (19.1) | 610 (18.4) | 666 (18.4) | 1896 (18.6) |
| BMI (kg/m2), mean (SD) | 29.4 (6.5) | 28.8 (27.9) | 29.2 (7.3) | 29.1 (7.0) |
| Blood pressure, mean (SD) | ||||
| SBP (mmHg) | 115.7 (29.1) | 124.2 (18.0) | 124 (17.7) | 121.8 (22.5) |
| DBP (mmHg) | 66.5 (18.5) | 72.3 (11.3) | 71.4 (11.1) | 70.1 (14.2) |
| Periodontitis, n (%) | 2065 (63.8) | 2076 (62.5) | 1900 (52.5) | 6041 (59.4) |
| Missing teeth, mean (SD) | 6.3 (6.1) | 5.4 (6.3) | 5.5 (6.2) | 5.7 (6.2) |
| DII, mean (SD) | −0.27 (1.80) | −0.21 (1.82) | −0.53 (1.98) | −0.35 (1.88) |
| Blood levels, mean (SD) | ||||
| WBC (109/L) | 6.7 (3.1) | 6.6 (2.4) | 7.1 (2.5) | 6.9 (2.6) |
| Segmented neutrophils (109/L) | 4.0 (2.6) | 3.94 (1.8) | 4.2 (1.8) | 4.1 (1.9) |
| Hba1c (%) | 5.6 (1.6) | 5.6 (1.6) | 5.7 (1.3) | 5.7 (1.4) |
| Vitamin D (mg/dL) | 64.5 (25.6) | 62.8 (30.9) | 67.1 (28.7) | 64.7 (28.7) |
| Total Cholesterol | 200.2 (40.0) | 186.9 (60.6) | 190.0 (49.9) | 191.5 (53.1) |
| CRP (mg/dL) | 0.39 (0.74) | - | - | - |
| NHANES 2009–2014 (n = 10,178) | NHANES 2009–2010 (n = 3238) | |
|---|---|---|
| Variable | DII | CRP |
| Mean PPD (mm) | −0.047 ** | 0.055 ** |
| Mean CAL (mm) | −0.042 ** | 0.062 ** |
| n sites PPD ≥ 5 mm (%) | −0.039 ** | 0.070 ** |
| n sites PPD ≥ 7 mm (%) | −0.030 ** | 0.056 ** |
| n sites CAL ≥ 5 mm (%) | −0.037 ** | 0.046 ** |
| n sites CAL ≥ 7 mm (%) | −0.030 ** | 0.068 ** |
| WBC (109/L) | −0.046 ** | 0.221 ** |
| Segmented neutrophils (109/L) | −0.039 ** | 0.232 ** |
| DII | - | 0.103 ** |
| NHANES 2009—2014 (n = 10,178) | Mean PPD (mm) | Mean CAL (mm) | n sites PPD ≥ 5 mm (%) | n sites CAL ≥ 5 mm (%) |
|---|---|---|---|---|
| Model 1 | 0.02 (0.02) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 2 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 3 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 4 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 5 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 6 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 7 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) *** | 0.00 (0.00) *** |
| Model 8 | 0.02 (0.00) *** | −0.02 (0.01) *** | 0.00 (0.00) ** | 0.00 (0.00) *** |
| Path a | Path b | Path c (Direct Effect) | Mediated Effect | Total Effect | Proportion Mediated (%) | |
|---|---|---|---|---|---|---|
| Exposure: Mean PPD | ||||||
| WBC (109/L) | 0.14 (0.04) *** | 0.04 (0.02) *** | 0.21 (0.06) *** | 0.01 (0.00) | 0.21 (0.06) *** | 2.7 |
| Segmented Neutrophils (109/L) | 0.14 (0.04) *** | 0.04 (0.02) *** | 0.15 (0.04) *** | 0.01 (0.00) | 0.16 (0.04) *** | 3.5 |
| Exposure: Mean CAL | ||||||
| WBC (109/L) | 0.10 (0.03) *** | 0.04 (0.02) *** | 0.17 (0.04) *** | 0.01 (0.00) | 0.17 (0.04) *** | 2.4 |
| Segmented Neutrophils (109/L) | 0.10 (0.03) *** | 0.03 (0.02) * | 0.13 (0.03) *** | 0.01 (0.00) | 0.14 (0.03) *** | 2.1 |
| Path a | Path b | Path c (Direct Effect) | Mediated Effect | Total Effect | Proportion Mediated (%) | |
|---|---|---|---|---|---|---|
| Exposure: Mean PPD | ||||||
| CRP (mg/dL) | 0.09 (0.07) | 0.04 (0.01) ** | 0.08 (0.04) | 0.01 (0.00) | 0.07 (0.04) * | NA |
| Exposure: Mean CAL | ||||||
| CRP (mg/dL) | 0.13 (0.05) * | 0.04 (0.01) ** | 0.06 (0.03) * | 0.01 (0.00) | 0.07 (0.03) ** | 52.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, V.; Botelho, J.; Viana, J.; Pereira, P.; Lopes, L.B.; Proença, L.; Delgado, A.S.; Mendes, J.J. Association between Dietary Inflammatory Index and Periodontitis: A Cross-Sectional and Mediation Analysis. Nutrients 2021, 13, 1194. https://doi.org/10.3390/nu13041194
Machado V, Botelho J, Viana J, Pereira P, Lopes LB, Proença L, Delgado AS, Mendes JJ. Association between Dietary Inflammatory Index and Periodontitis: A Cross-Sectional and Mediation Analysis. Nutrients. 2021; 13(4):1194. https://doi.org/10.3390/nu13041194
Chicago/Turabian StyleMachado, Vanessa, João Botelho, João Viana, Paula Pereira, Luísa Bandeira Lopes, Luís Proença, Ana Sintra Delgado, and José João Mendes. 2021. "Association between Dietary Inflammatory Index and Periodontitis: A Cross-Sectional and Mediation Analysis" Nutrients 13, no. 4: 1194. https://doi.org/10.3390/nu13041194