Milk Consumption and Respiratory Function in Asthma Patients: NHANES Analysis 2007–2012
Abstract
:1. Introduction
2. Materials and Methods
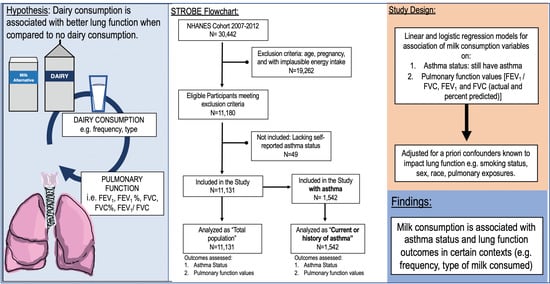
2.1. Study Design
2.2. Participants
2.3. Variables
2.4. Statistical Methods
3. Results
3.1. Descriptive Data of Eligible Participants among the Nhanes Cohort
3.2. Milk Consumption Tendencies and Lung Function Measurements in All Eligible Participants
3.3. Milk Consumption Tendencies and Current Asthma Report in All Eligible Participants
3.4. Milk Consumption Tendencies and Current Asthma Report in Participants Reporting Asthma (History or Current)
3.5. Milk Consumption Tendencies and Lung Function Parameters in Participants Reporting Asthma (History or Current)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viegi, G.; Maio, S.; Fasola, S.; Baldacci, S. Global Burden of Chronic Respiratory Diseases. J. Aerosol. Med. Pulm. Drug Deliv. 2020, 33, 171–177. [Google Scholar] [CrossRef]
- Syamlal, G.; Bhattacharya, A.; Dodd, K.E. Medical Expenditures Attributed to Asthma and Chronic Obstructive Pulmonary Disease Among Workers—United States, 2011–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 809–814. [Google Scholar] [CrossRef]
- Rai, D.K.; Sharma, P.; Kumar, R. Post covid 19 pulmonary fibrosis—Is it reversible? Indian J. Tuberc. 2020. [Google Scholar] [CrossRef]
- McGeachie, M. Childhood asthma is a risk factor for the development of chronic obstructive pulmonary disease. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 104–109. [Google Scholar] [CrossRef]
- Silva, G.; Sherrill, D.; Guerra, S.; Barbee, R. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004, 126, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Adibi, A.; Safari, A.; FitzGerald, J.M.; Sadatsafavi, M. The Projected Economic and Health Burden of Uncontrolled Asthma in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Shah, K.S.; Shallcross, M.L. A qualitative study of physician perspectives of cost-related communication and patients’ financial burden with managing chronic disease. BMC Health Serv. Res. 2015, 15, 518. [Google Scholar] [CrossRef] [Green Version]
- Jamison, D.T.; Gelband, H.; Horton, S.; Jha, P.; Laxminarayan, R.; Mock, C.N.; Nugent, R. (Eds.) Disease Control Priorities: Improving Health and Reducing Poverty. In Chapter 13: Strengthening Health Systems to Provide Emergency Care; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Braido, F. Failure in asthma control: Reasons and consequences. Scientifica 2013, 2013, 549252. [Google Scholar] [CrossRef] [Green Version]
- Bridgeman, M.B.; Wilken, L.A. Essential Role of Pharmacists in Asthma Care and Management. J. Pharm. Pract. 2021, 34, 149–162. [Google Scholar] [CrossRef]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. [Google Scholar] [CrossRef] [Green Version]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western diet pattern and adult asthma: A focused review. Ann Allergy Asthma Immunol 2015, 114, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.G. Diet, Obesity, and Asthma. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S5), S332–S338. [Google Scholar] [CrossRef]
- Lindemann, J.; David Pampe, E.; Peterkin, J.J.; Orozco-Cronin, P.; Belofsky, G.; Stull, D. Clinical study of the effects on asthma-related QOL and asthma management of a medical food in adult asthma patients. Curr. Med. Res. Opin. 2009, 25, 2865–2875. [Google Scholar] [CrossRef]
- Surette, M.E.; Stull, D.; Lindemann, J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr. Med. Res. Opin. 2008, 24, 559–567. [Google Scholar] [CrossRef]
- Cornell, K.; Alam, M.; Lyden, E.; Wood, L.; LeVan, T.D.; Nordgren, T.M.; Bailey, K.; Hanson, C. Saturated Fat Intake Is Associated with Lung Function in Individuals with Airflow Obstruction: Results from NHANES 2007–2012. Nutrients 2019, 11, 317. [Google Scholar] [CrossRef] [Green Version]
- Hanson, C.; Sayles, H.; Rutten, E.; Wouters, E.F.M.; MacNee, W.; Calverley, P.; Meza, J.L.; Rennard, S. The Association Between Dietary Intake and Phenotypical Characteristics of COPD in the ECLIPSE Cohort. Chronic. Obstr. Pulm. Dis. 2014, 1, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Waser, M.; Michels, K.B.; Bieli, C.; Floistrup, H.; Pershagen, G.; von Mutius, E.; Ege, M.; Riedler, J.; Schram-Bijkerk, D.; Brunekreef, B.; et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin. Exp. Allergy 2007, 37, 661–670. [Google Scholar] [CrossRef]
- Jiang, R.; Jacobs, D.R.; He, K.; Hoffman, E.; Hankinson, J.; Nettleton, J.A.; Barr, R.G. Associations of dairy intake with CT lung density and lung function. J. Am. Coll. Nutr. 2010, 29, 494–502. [Google Scholar] [CrossRef] [Green Version]
- National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Available online: https://www.cdc.gov/nchs/index.htm (accessed on 31 March 2021).
- Divya, M.S.; Roshin, G.E.; Divya, T.S.; Rasheed, V.A.; Santhoshkumar, T.R.; Elizabeth, K.E.; James, J.; Pillai, R.M. Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors co-expressing mesenchymal stem cell and neuronal markers capable of instantaneous neuronal differentiation. Stem Cell Res. Ther. 2012, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Lambert, A.; Drummond, M.B.; Wei, C.; Irvin, C.; Kaminsky, D.; McCormack, M.; Wise, R. Diagnostic accuracy of FEV1/forced vital capacity ratio z scores in asthmatic patients. J. Allergy Clin. Immunol. 2015, 136, 649–653.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthonisen, N.R.; Connett, J.E.; Murray, R.P. Smoking and lung function of Lung Health Study participants after 11 years. Am. J. Respir. Crit. Care Med. 2002, 166, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, M.J.; Crapo, R.O. Socioeconomic status and lung function. Chest 2007, 132, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.E.; Peters, U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkotter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution exposure. ESCAPE: A multicentre cohort study and meta-analysis. Eur. Respir. J. 2015, 45, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamzah, N.A.; Tamrin, S.B.M.; Ismail, N.H. Metal dust exposure and lung function deterioration among steel workers: An exposure-response relationship. Int. J. Occup. Environ. Health 2016, 22, 224–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querol, X.; Tobias, A.; Perez, N.; Karanasiou, A.; Amato, F.; Stafoggia, M.; Perez Garcia-Pando, C.; Ginoux, P.; Forastiere, F.; Gumy, S.; et al. Monitoring the impact of desert dust outbreaks for air quality for health studies. Environ. Int. 2019, 130, 104867. [Google Scholar] [CrossRef]
- Ferrante, G.; La Grutta, S. The Burden of Pediatric Asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef] [Green Version]
- Labonte, M.E.; Couture, P.; Richard, C.; Desroches, S.; Lamarche, B. Impact of dairy products on biomarkers of inflammation: A systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am. J. Clin. Nutr. 2013, 97, 706–717. [Google Scholar] [CrossRef] [Green Version]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- Stancliffe, R.A.; Thorpe, T.; Zemel, M.B. Dairy attentuates oxidative and inflammatory stress in metabolic syndrome. Am. J. Clin. Nutr. 2011, 94, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zhou, Q.; Kwame Amakye, W.; Su, Y.; Zhang, Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: A systematic review and meta analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1122–1130. [Google Scholar] [CrossRef]
- House, J.; Wyss, A.; Hoppin, J.; Richards, M.; Long, S.; Umbach, D.; Henneberger, P.; Beane, F.L.; Sandler, D.; Long, O.C.E.; et al. Early-life farm exposures and adult asthma and atopy in the Agricultural Lung Health Study. J. Allergy Clin. Immunol. 2017, 140, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Wyss, A.B.; House, J.S.; Hoppin, J.A.; Richards, M.; Hankinson, J.L.; Long, S.; Henneberger, P.K.; Freeman, L.E.; Sandler, D.P.; O’Connell, E.L.; et al. Raw milk consumption and other early-life farm exposures and adult pulmonary function in the Agricultural Lung Health Study. Thorax 2018, 73, 279–282. [Google Scholar] [CrossRef]
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773. [Google Scholar] [CrossRef]
- Summer, A.; Lora, I.; Formaggioni, P.; Gottardo, F. Impact of heat stress on milk and meat production. Anim. Front. 2018, 9, 39–46. [Google Scholar] [CrossRef]
- Wankar, A.; Rindhe, S.; Doijad, N. Heat stress in dairy animals and current milk production trends, economics, and future perspectives: The global scenario. Trop. Anim. Health Prod. 2021, 53, 70. [Google Scholar] [CrossRef]
- Liu, Z.; Ezernieks, V.; Wang, J.; Arachchillage, N.W.; Garner, J.B.; Wales, W.J.; Cocks, B.G.; Rochfort, S. Heat Stress in Dairy Cattle Alters Lipid Composition of Milk. Sci. Rep. 2017, 7, 961. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Lee, E.; Lee, I.H.; Lee, S.R. Effects of transport stress on physiological responses and milk production in lactating dairy cows. Asian-Australas J. Anim. Sci. 2019, 32, 442–451. [Google Scholar] [CrossRef]
- Chamberlain, A.J.; Hayes, B.J.; Savin, K.; Bolormaa, S.; McPartlan, H.C.; Bowman, P.J.; Van der Jagt, C.; MacEachern, S.; Goddard, M.E. Validation of single nucleotide polymorphisms associated with milk production traits in dairy cattle. J. Dairy Sci. 2012, 95, 864–875. [Google Scholar] [CrossRef] [Green Version]
- Raschia, M.A.; Nani, J.P.; Maizon, D.O.; Beribe, M.J.; Amadio, A.F.; Poli, M.A. Single nucleotide polymorphisms in candidate genes associated with milk yield in Argentinean Holstein and Holstein x Jersey cows. J. Anim. Sci. Technol. 2018, 60, 31. [Google Scholar] [CrossRef] [Green Version]
- Melzer, N.; Wittenburg, D.; Repsilber, D. Integrating milk metabolite profile information for the prediction of traditional milk traits based on SNP information for Holstein cows. PLoS ONE 2013, 8, e70256. [Google Scholar] [CrossRef] [Green Version]
- Illi, S.; Depner, M.; Genuneit, J.; Horak, E.; Loss, G.; Strunz-Lehner, C.; Buchele, G.; Boznanski, A.; Danielewicz, H.; Cullinan, P.; et al. Protection from childhood asthma and allergy in Alpine farm environments—The GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 2012, 129, 1470–1477.e6. [Google Scholar] [CrossRef]
- Berthon, B.S.; Wood, L.G. Nutrition and respiratory health—Feature review. Nutrients 2015, 7, 1618–1643. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Berry, L.J.; Elliot, J.G.; James, A.L.; Gorman, S.; Hart, P.H. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am. J. Respir. Crit. Care Med. 2011, 183, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Celedon, J.C.; Soto-Quiros, M.E.; Avila, L.; Hunninghake, G.M.; Forno, E.; Laskey, D.; Sylvia, J.S.; Hollis, B.W.; Weiss, S.T.; et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am. J. Respir. Crit. Care Med. 2009, 179, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, Y.Q.; Yin, J.; Yao, J.; Shen, J.; Sheng, G.J.; Li, K.; Lv, H.F.; Fang, X.; Wu, W.F. Meta-analysis of vitamin D and lung function in patients with asthma. Respir. Res. 2019, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Damera, G.; Fogle, H.W.; Lim, P.; Goncharova, E.A.; Zhao, H.; Banerjee, A.; Tliba, O.; Krymskaya, V.P.; Panettieri, R.A., Jr. Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br. J. Pharmacol. 2009, 158, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Mansson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Brick, T.; Schober, Y.; Bocking, C.; Pekkanen, J.; Genuneit, J.; Loss, G.; Dalphin, J.C.; Riedler, J.; Lauener, R.; Nockher, W.A.; et al. omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow’s milk. J. Allergy Clin. Immunol. 2016, 137, 1699–1706.e13. [Google Scholar] [CrossRef] [Green Version]
- Lluis, A.; Depner, M.; Gaugler, B.; Saas, P.; Casaca, V.I.; Raedler, D.; Michel, S.; Tost, J.; Liu, J.; Genuneit, J.; et al. Protection Against Allergy: Study in Rural Environments Study, G., Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J. Allergy Clin. Immunol. 2014, 133, 551–559. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Heires, A.J.; Zempleni, J.; Swanson, B.J.; Wichman, C.; Romberger, D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019, 64, 110–120. [Google Scholar] [CrossRef]
- Van Neerven, R.J.; Knol, E.F.; Heck, J.M.; Savelkoul, H.F. Which factors in raw cow’s milk contribute to protection against allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Johnson, J.D.; Theurer, W.M. A stepwise approach to the interpretation of pulmonary function tests. Am. Fam. Physician 2014, 89, 359–366. [Google Scholar]
- Thiara, G.; Goldman, R.D. Milk consumption and mucus production in children with asthma. Can. Fam. Physician 2012, 58, 165–166. [Google Scholar]
- Bartley, J.; McGlashan, S.R. Does milk increase mucus production? Med. Hypotheses 2010, 74, 732–734. [Google Scholar] [CrossRef]
- Wuthrich, B.; Schmid, A.; Walther, B.; Sieber, R. Milk consumption does not lead to mucus production or occurrence of asthma. J. Am. Coll. Nutr. 2005, 24 (Suppl. S6), 547S–555S. [Google Scholar] [CrossRef]


| Consumption Frequency Variables | Definition of Variable | Response Categories |
|---|---|---|
| Lifetime regular milk drinker (REGMLK) | Milk consumption, regardless of type, 5+ days per week for a lifetime | Lifetime: most of their life Varied: sometimes a regular milk drinker Never: never a regular milk drinker |
| Dichotomized lifetime regular milk drinker (REGMLKR) | Milk consumption, regardless of type, 5+ days per week for a lifetime dichotomized as Lifetime/Varied or Never | Yes (Lifetime) No (Varied/Never) |
| Any type milk consumption within prior 30 days (MLKCONS30) | Frequency of milk consumption, regardless of type, in the 30 days prior to survey date | Daily: 1+ times daily Sometimes: 1+ times a week but not daily Rarely: less than 1 time per week Never: no milk consumption |
| Dichotomized any type milk consumption within prior 30 days (MLKCONS30R) | Frequency of milk consumption, regardless of type, in the 30 days prior to survey date, dichotomized as 5+ times a week or Sometimes/Rarely/Never | Yes (5+ times a week or more) No (Sometimes/Rarely/Never) |
| Type of Milk Consumption Variables | Definition of Variable | Response Categories |
| Exclusive milk consumed by type 5+ days per week in prior 30 days (TYPE 30) | Whole (full fat) only, 2% (reduced fat) only, 1% (low fat) only, skim (fat free) only, other type of milk (non-dairy, such as soy) only, 5+ days per week in last 30 days | Yes No |
| Characteristic | ALL (n = 11,131) | No History of Asthma (n = 9589) | Yes, Have a History of Asthma (n = 717) | Yes, Still Have Asthma (n = 825) | p-Value |
|---|---|---|---|---|---|
| Mean or % | Mean or % | Mean or % | Mean or % | ||
| Age (year) | 44.4 | 44.8 | 40.2 | 44.0 | <0.0001 |
| Gender | <0.0001 | ||||
| Female | 51.0% | 49.6% | 53.4% | 64.2% | |
| Male | 49.0% | 50.4% | 46.6% | 35.8% | |
| Race | <0.0001 | ||||
| Hispanic | 13.6% | 14.2% | 11.1% | 8.7% | |
| Non-Hispanic White | 69.9% | 69.5% | 70.6% | 74.0% | |
| Non-Hispanic Black | 10.3% | 10.0% | 11.9% | 12.8% | |
| Other Race | 6.2% | 6.3% | 6.4% | 4.5% | |
| BMI group | 0.0002 | ||||
| Underweight/Normal: < 25.0 | 31.5% | 32.0% | 31.4% | 26.4% | |
| Overweight: 25.0–29.9 | 33.5% | 34.1% | 29.0% | 30.2% | |
| Obesity: > 30 | 35.0% | 33.9% | 39.6% | 43.4% | |
| Smoking | 0.250 | ||||
| Non-Smoker | 54.8% | 55.2% | 54.9% | 50.4% | |
| Former Smoker | 23.8% | 23.7% | 23.7% | 24.5% | |
| Smoker | 21.4% | 21.1% | 21.4% | 25.1% | |
| Poverty | 0.0004 | ||||
| Poor | 21.4% | 20.7% | 22.8% | 28.0% | |
| Nearly poor | 8.8% | 8.6% | 8.9% | 10.6% | |
| Not poor | 69.8% | 70.6% | 68.3% | 61.4% | |
| Education | 0.230 | ||||
| Less than 12th grade | 15.8% | 16.0% | 14.6% | 14.4% | |
| High school/GED | 22.0% | 22.0% | 20.7% | 23.5% | |
| Some college or AA | 32.0% | 31.4% | 34.7% | 35.6% | |
| College or above | 30.2% | 30.5% | 30.1% | 26.5% | |
| Mine dust | 0.480 | ||||
| No | 68.2% | 68.0% | 70.3% | 69.7% | |
| Yes | 31.7% | 32.0% | 29.7% | 30.3% | |
| Natural dust | 0.500 | ||||
| No | 76.9% | 79.2% | 81.1% | 75.7% | |
| Yes | 23.1% | 20.8% | 18.9% | 24.3% | |
| Regular Milk Drinker | 0.049 | ||||
| Never been | 21.3% | 21.2% | 20.9% | 23.1% | |
| Sometimes/varied | 35.8% | 35.8% | 31.6% | 38.8% | |
| Lifetime | 42.9% | 43.0% | 47.5% | 38.1% | |
| Lifetime regular milk drinker (Yes/No) | 0.016 | ||||
| Yes | 45.2% | 46.0% | 38.5% | 41.4% | |
| No | 54.8% | 54.0% | 61.5% | 58.6% | |
| Any type milk consumption (30 days) | 0.410 | ||||
| Never | 15.8% | 15.7% | 14.3% | 18.2% | |
| Rarely: < once a week | 15.4% | 15.5% | 13.8% | 15.9% | |
| Sometimes: < once a day | 29.8% | 30.0% | 28.7% | 28.3% | |
| Often: once a day or more | 39.0% | 68.8% | 43.2% | 37.6% | |
| Any type milk consumption 5X a week or more (30 days) | 0.160 | ||||
| Yes | 61.0% | 61.2% | 56.8% | 62.4% | |
| No | 39.0% | 38.8% | 43.2% | 37.6% | |
| Exclusive type milk consumed (30 days) | 0.720 | ||||
| Never | 15.8% | 15.7% | 14.3% | 18.2% | |
| Regular/whole milk only | 17.4% | 17.3% | 19.0% | 16.3% | |
| 2% milk only | 31.5% | 31.4% | 30.9% | 33.1% | |
| 1% milk only | 12.0% | 12.1% | 10.4% | 11.1% | |
| Skim milk only | 16.5% | 16.4% | 17.5% | 15.7% | |
| Other milk only | 3.9% | 4.0% | 3.6% | 2.6% | |
| 2+ types milk combined | 3.1% | 3.0% | 4.2% | 3.0% | |
| Baseline FEV1 (mL) | 3245.6 | 3273.9 | 3317.1 | 2868.2 | <0.0001 |
| Baseline FVC (mL) | 4155.4 | 4155.4 | 4221.7 | 3841.3 | <0.0001 |
| FEV1% predicted | 96.3 | 97.1 | 95.3 | 88.7 | <0.0001 |
| FVC % predicted | 99.1 | 99.5 | 98.8 | 96.0 | <0.0001 |
| FEV1/FVC | 0.78 | 0.78 | 0.78 | 0.75 | <0.0001 |
| Milk Consumption Tendencies (n = 11,180) | FEV1 | FVC | FEV1% | FVC% | FEV1/FVC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 × 10 3 [SE × 103] 2 | p3 | |
| Regular Milk Drinker (REGMLK vs. Never) | ≤0.01 | 0.02 | 0.17 | 0.29 | 0.69 | |||||
| Lifetime | 54.54 [16.0] | ≤0.01 | 57.71 [21.4] | ≤0.01 | 0.59 [0.44] | 0.19 | 0.32 [0.39] | 0.42 | 1.78 [2.54] | 0.49 |
| Variable/Sometimes | 58.42 [20.0] | ≤0.01 | 58.77 [22.3] | ≤0.01 | 1.05 [0.54] | 0.06 | 0.64 [0.38] | 0.10 | 3.04 [3.48] | 0.39 |
| 30-Day Milk Consumption Frequency (MLKCONS30 vs. Never) | 0.20 | 0.32 | 0.35 | 0.47 | 0.36 | |||||
| Rarely | 26.34 [23.0] | 0.26 | 13.88 [25.0] | 0.58 | 0.95 [0.63] | 0.14 | 0.40 [0.50] | 0.43 | 4.18 [2.87] | 0.63 |
| Sometimes | 30.44 [23.7] | 0.20 | 22.11 [27.6] | 0.42 | 0.97 [0.61] | 0.11 | 0.64 [0.50] | 0.21 | 3.60 [3.07] | 0.15 |
| Often | 39.55 [18.3] | 0.04 | 40.93 [22.4] | 0.07 | 0.92 [0.57] | 0.12 | 0.73 [0.48] | 0.14 | 1.40 [2.90] | 0.25 |
| Regular 30-day Exclusive Type Milk Consumption (TYPE30 vs. Never) | 0.14 | 0.30 | 0.16 | 0.07 | 0.11 | |||||
| Whole | −1.10 [21.7] | 0.96 | −5.93 [24.1] | 0.81 | 0.29 [0.58] | 0.62 | 0.12 [0.47] | 0.79 | 0.44 [3.29] | 0.32 |
| 2% | 36.34 [19.8] | 0.07 | 37.02 [24.4] | 0.14 | 0.92 [0.56] | 0.11 | 0.81 [0.45] | 0.08 | 1.72 [3.01] | 0.57 |
| 1% | 59.50 [32.7] | 0.08 | 52.02 [39.2] | 0.19 | 1.81 [0.75] | 0.02 | 1.27 [0.62] | 0.05 | 5.07 [3.15] | 0.11 |
| Skim | 50.41 [25.5] | 0.05 | 45.96 [29.4] | 0.12 | 1.28 [0.74] | 0.09 | 0.83 [0.60] | 0.18 | 3.60 [3.61] | 0.32 |
| Other | 30.50 [35.6] | 0.40 | 12.63 [44.8] | 0.78 | 0.65 [0.99] | 0.51 | −0.03 [0.94] | 0.97 | 6.38 [3.56] | 0.08 |
| 2+ types | 32.72 [42.7] | 0.45 | 6.15 [48.4] | 0.90 | 0.09 [1.01] | 0.93 | −0.59 [0.91] | 0.52 | 6.28 [4.93] | 0.20 |
| Characteristic | Current Asthma | Current Asthma |
|---|---|---|
| Yes vs. No | Yes vs. No | |
| Univariable | Multivariable | |
| p value 1 | p value 1 | |
| OR 2 [95% CI] 3 | OR 2 [95% CI] 3 | |
| Age (year) | p < 0.001 | p = 0.001 |
| 1.02 [1.01, 1.02] | 1.02 [1.01, 1.03] | |
| Gender | p = 0.001 | p = 0.029 |
| Female | 1.56 [1.21, 2.02] | 1.43 [1.04, 1.96] |
| Male | 1 | 1 |
| Race | p = 0.107 | p = 0.319 |
| Hispanic | 0.74 [0.52, 1.07] | 0.74 [0.51, 1.08] |
| Non-Hispanic White | 1 | 1 |
| Non-Hispanic Black | 1.03 [0.76, 1.40] | 0.96 [0.68, 1.37] |
| Other race | 0.67 [0.42, 1.06] | 0.71 [0.40, 1.25] |
| BMI group | p = 0.229 | p = 0.893 |
| Underweight/Normal: > 25.0 | 1 | 1 |
| Overweight: 25.0-29.9 | 1.25 [0.87, 1.78] | 1.10 [0.75, 1.61] |
| Obesity: > 30 | 1.30 [0.96, 1.77] | 1.08 [0.73, 1.60] |
| Smoking | p = 0.257 | p = 0.600 |
| Non-smoker | 1 | 1 |
| Former smoker | 1.13 [0.83, 1.54] | 0.99 [0.69, 1.41] |
| Smoker | 1.28 [0.96, 1.70] | 1.17 [0.85, 1.62] |
| Poverty | p = 0.120 | p = 0.044 |
| Poor | 1.37 [1.00, 1.87] | 1.47 [1.04, 2.08] |
| Nearly poor | 1.32 [0.84, 2.10] | 1.41 [0.92, 2.14] |
| Not poor | 1 | 1 |
| Education | p = 0.652 | p = 0.637 |
| Less than 12th grade | 1 | 1 |
| High school/GED | 1.15 [0.76, 1.75] | 1.34 [0.82, 2.18] |
| Some college or AA | 1.04 [0.71, 1.52] | 1.29 [0.83, 2.00] |
| College or above | 0.89 [0.60, 1.33] | 1.13 [0.71, 1.81] |
| Mine dust | p = 0.791 | p = 0.085 |
| No | 1 | 1 |
| Yes | 1.03 [0.83, 1.28] | 1.27 [0.97, 1.67] |
| Natural dust | p = 0.083 | 0.051 |
| No | 1 | 1 |
| Yes | 0.83 [0.66, 1.03] | 0.77 [0.59, 1.00] |
| Regular milk drinker | p = 0.002 | p = 0.006 |
| Lifetime | 0.73 [0.57, 0.93] | 0.75 [0.56, 1.01] |
| Sometimes/Varied | 1.12 [0.78, 1.60] | 1.13 [0.76, 1.69] |
| Never been | 1 | 1 |
| Milk Consumption Tendencies | FEV1 | FVC | FEV1% | FVC% | FEV1/FVC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1538) | β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 [SE] 2 | p3 | β 1 × 103 [SE × 103] 2 | p3 |
| Regular Milk Drinker (REGMLK vs. Never) | 0.38 | 0.06 | 0.38 | 0.05 | 0.46 | |||||
| Lifetime | 21.49 [51.0] | 0.68 | 67.81 [62.0] | 0.28 | 0.39 [1.59] | 0.81 | 1.34 [1.34] | 0.32 | −9.72 [7.99] | 0.23 |
| Variable/Sometimes | 60.61 [51.7] | 0.25 | 127.282 [56.8] | 0.03 | 1.64 [1.38] | 0.24 | 2.62 [1.08] | 0.02 | −7.83 [7.64] | 0.31 |
| 30-Day Milk Consumption Frequency (MLKCONS30 vs. Never) | 0.26 | 0.45 | 0.25 | 0.56 | 0.47 | |||||
| Rarely | 106.54 [64.4] | 0.10 | 116.10 [75.3] | 0.13 | 2.93 [1.62] | 0.08 | 2.23 [1.55] | 0.16 | 2.25 [9.31] | 0.20 |
| Sometimes | 44.76 [64.0] | 0.49 | 69.63 [67.8] | 0.31 | 0.94 [1.60] | 0.56 | 1.31 [1.25] | 0.30 | −1.75 [7.37] | 0.81 |
| Often | 21.08 [60.9] | 0.73 | 60.32 [69.5] | 0.39 | 0.48 [1.75] | 0.78 | 1.17 [1.45] | 0.42 | −8.77 [6.72] | 0.81 |
| Regular 30-day Exclusive Type Milk Consumption (TYPE30 vs. Never) | 0.08 | ≤0.01 | 0.22 | ≤0.01 | 0.54 | |||||
| Whole | −78.40 [51.6] | 0.14 | −76.80 [62.9] | 0.23 | −1.71 [1.47] | 0.25 | −1.22 [1.27] | 0.34 | −9.56 [8.95] | 0.29 |
| 2% | 67.54 [56.7] | 0.24 | 108.14 [64.8] | 0.10 | 1.02 [1.45] | 0.48 | 1.44 [1.29] | 0.27 | −3.58 [5.54] | 0.52 |
| 1% | 114.60 [78.6] | 0.15 | 144.53 [90.8] | 0.12 | 4.24 [2.36] | 0.08 | 4.20 [2.14] | 0.06 | 0.46 [9.63] | 0.96 |
| Skim | 86.53 [72.2] | 0.24 | 138.04 [79.9] | 0.09 | 2.07 [1.75] | 0.24 | 2.76 [1.34] | 0.05 | −6.96 [8.21] | 0.40 |
| Other | 83.76 [92.1] | 0.37 | 51.15 [93.7] | 0.59 | 0.26 [2.51] | 0.92 | −1.21 [2.25] | 0.59 | 9.10 [13.5] | 0.50 |
| 2+ types | 15.82 [98.7] | 0.87 | 21.34 [119.7] | 0.86 | 2.00 [3.10] | 0.48 | 1.73 [2.83] | 0.54 | 1.37 [10.7] | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sveiven, S.N.; Bookman, R.; Ma, J.; Lyden, E.; Hanson, C.; Nordgren, T.M. Milk Consumption and Respiratory Function in Asthma Patients: NHANES Analysis 2007–2012. Nutrients 2021, 13, 1182. https://doi.org/10.3390/nu13041182
Sveiven SN, Bookman R, Ma J, Lyden E, Hanson C, Nordgren TM. Milk Consumption and Respiratory Function in Asthma Patients: NHANES Analysis 2007–2012. Nutrients. 2021; 13(4):1182. https://doi.org/10.3390/nu13041182
Chicago/Turabian StyleSveiven, Stefanie N., Rachel Bookman, Jihyun Ma, Elizabeth Lyden, Corrine Hanson, and Tara M. Nordgren. 2021. "Milk Consumption and Respiratory Function in Asthma Patients: NHANES Analysis 2007–2012" Nutrients 13, no. 4: 1182. https://doi.org/10.3390/nu13041182