Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors
Abstract
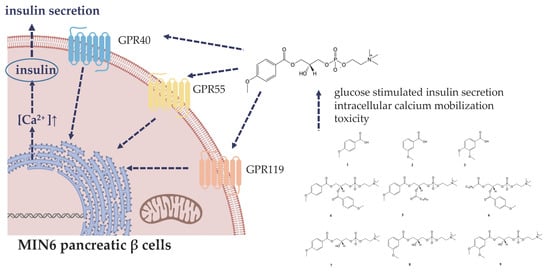
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of 1-(3-Methoxy)Benzoyl-2-Hydroxy-Sn-Glycero-3-Phosphocholine (8)
2.3. MIN6 Cell Line Culture
2.4. Cell Viability
2.5. Glucose-Stimulated Insulin Secretion (GSIS)
2.6. Calcium Flux Measurements
2.7. Statistical Analysis
3. Results
3.1. Synthesis of Phospholipids Containing Methoxy Derivatives of Benzoic Acid
3.2. The Influence of Conjugates of Phosphatidylcholine and Lysophosphatidylcholine with p-Anisic Acid on MIN6 Viability
3.3. The Influence of Conjugates of Phosphatidylcholine and Lysophosphatidylcholine with p-Anisic Acid on GSIS and Intracellular Ca2+ Mobilization in MIN6 cells
3.4. The Influence of Conjugates of Lysophosphatidylcholine with Veratric and 3-Methoxybenzoic Acid on Viability, GSIS and Intracellular Ca2+ Mobilization in MIN6 Cells
3.5. The Role of GPR40, GPR55, and GPR119 in GSIS and Intracellular Ca2+ Mobilization Induced by 1-Anisoyl-2-Hydroxy-Sn-Glycero-3-Phosphocholine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157. [Google Scholar] [CrossRef] [Green Version]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [Green Version]
- Belete, T.M. A recent achievement in the discovery and development of novel targets for the treatment of type-2 diabetes mellitus. J. Exp. Pharmacol. 2020, 12, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanjan, M.J.; Mohammed, M.; Prashantha Kumar, B.R.; Chandrasekar, M.J.N. Thiazolidinediones as antidiabetic agents: A critical review. Bioorganic Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Prasad-Reddy, L.; Isaacs, D. A clinical review of GLP-1 receptor agonists: Efficacy and safety in diabetes and beyond. Drugs Context 2015, 4, 212283. [Google Scholar] [CrossRef]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorganic Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef]
- Hücking, K.; Kostic, Z.; Pox, C.; Ritzel, R.; Holst, J.J.; Schmiegel, W. α-glucosidase inhibition (acarbose) fails to enhance secretion of glucagon-like peptide 1 (7–36 amide) and to delay gastric emptying in Type 2 diabetic patients. Diabet. Med. 2005, 22, 470–476. [Google Scholar] [CrossRef]
- Singh, R.; Kazmi, I.; Afzal, M.; Imam, F.; Alharbi, K.S. Dietary Phytochemicals and Their Potential Effects on Diabetes Mellitus 2. In Plant and Human Health; Springer International Publishing: Basel, Switzerland, 2019; Volume 3, pp. 65–86. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, P.; Bomzan, D.P.; Sathyendra Rao, B.V.; Sreerama, Y.N. Distribution of phenolic antioxidants in whole and milled fractions of quinoa and their inhibitory effects on α-amylase and α-glucosidase activities. Food Chem. 2016, 199, 330–338. [Google Scholar] [CrossRef]
- Cherng, Y.-G.; Tsai, C.-C.; Chung, H.-H.; Lai, Y.-W.; Kuo, S.-C.; Cheng, J.-T. Antihyperglycemic action of sinapic acid in diabetic rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; Chatham, L.; Juvik, J.; Singh, V.; Somavat, P.; de Mejia, E.G. Activating Effects of Phenolics from Apache Red Zea mays L. on Free Fatty Acid Receptor 1 and Glucokinase Evaluated with a Dual Culture System with Epithelial, Pancreatic, and Liver Cells. J. Agric. Food Chem. 2019, 67, 9148–9159. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Mu, Y.; Chen, H.; Xiu, Z.; Yang, T. Enzymatic synthesis of feruloylated lysophospholipid in a selected organic solvent medium. Food Chem. 2013, 141, 3317–3322. [Google Scholar] [CrossRef]
- Anankanbil, S.; Pérez, B.; Banerjee, C.; Guo, Z. New phenophospholipids equipped with multi-functionalities: Regiospecific synthesis and characterization. J. Colloid Interface Sci. 2018, 523, 169–178. [Google Scholar] [CrossRef]
- Marrapu, B.; Ma, J.; Geng, Z.; Nalla, S.; Liu, F.; Li, P. Chemo-enzymatic synthesis, characterization, in vitro antioxidant capacity and oxidative stability studies of novel phosphatidylcholines with ω-3/ω-6 PUFAs and phenolic acids. Food Res. Int. 2020, 131, 109010. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, Characterization, and In Vitro Cancer Cell Growth Inhibition Evaluation of Novel Phosphatidylcholines with Anisic and Veratric Acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnecka, M.; Switalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis and biological evaluation of phosphatidylcholines with cinnamic and 3-methoxycinnamic acids with potent antiproliferative activity. RSC Adv. 2018, 8, 35744–35752. [Google Scholar] [CrossRef]
- Drzazga, A.; Kristinsson, H.; Sałaga, M.; Zatorski, H.; Koziołkiewicz, M.; Gendaszewska-Darmach, E. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell. Endocrinol. 2018, 472, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Drzazga, A.; Sowińska, A.; Krzemińska, A.; Okruszek, A.; Paneth, P.; Koziołkiewicz, M. 2-OMe-lysophosphatidylcholine analogues are GPR119 ligands and activate insulin secretion from βTC-3 pancreatic cells: Evaluation of structure-dependent biological activity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gendaszewska-Darmach, E.; Drzazga, A.; Koziołkiewicz, M. Targeting GPCRs Activated by Fatty Acid-Derived Lipids in Type 2 Diabetes. Trends Mol. Med. 2019, 25, 915–929. [Google Scholar] [CrossRef] [Green Version]
- Milligan, G.; Shimpukade, B.; Ulven, T.; Hudson, B.D. Complex Pharmacology of Free Fatty Acid Receptors. Chem. Rev. 2017, 117, 67–110. [Google Scholar] [CrossRef]
- Amisten, S.; Salehi, A.; Rorsman, P.; Jones, P.M.; Persaud, S.J. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 2013, 139, 359–391. [Google Scholar] [CrossRef]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Drzazga, A.; Sowinska, A.; Krzeminska, A.; Rytczak, P.; Koziolkiewicz, M.; Gendaszewska-Darmach, E. Lysophosphatidylcholine elicits intracellular calcium signaling in a GPR55-dependent manner. Biochem. Biophys. Res. Commun. 2017, 489, 242–247. [Google Scholar] [CrossRef]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Ghislain, J.; Poitout, V. The Role and Future of FFA1 as a Therapeutic Target. In Handbook of Experimental Pharmacology; Springer: Cham, Switherland, 2016; Volume 236, pp. 159–180. [Google Scholar] [CrossRef]
- Burant, C.F.; Viswanathan, P.; Marcinak, J.; Cao, C.; Vakilynejad, M.; Xie, B. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2012, 379, 1403–1411. [Google Scholar] [CrossRef]
- Briscoe, C.P.; Tadayyon, M.; Andrews, J.L.; Benson, W.G.; Chambers, J.K.; Eilert, M.M. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 2003, 278, 11303–11311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, H.S.; Rosenkilde, M.M.; Holst, J.J.; Schwartz, T.W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 2012, 33, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; O’Neill, K.; Lan, H.; Pang, L.; Shan, L.X.; Hawes, B.E. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br. J. Pharmacol. 2009, 155, 1056–1065. [Google Scholar] [CrossRef] [Green Version]
- Overton, H.A.; Fyfe, M.C.T.; Reynet, C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 2008, 153, S76–S81. [Google Scholar] [CrossRef] [Green Version]
- Moss, C.E.; Glass, L.L.; Diakogiannaki, E.; Pais, R.; Lenaghan, C.; Smith, D.M. Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides 2016, 77, 16–20. [Google Scholar] [CrossRef]
- Henstridge, C.M.; Balenga, N.A.B.; Kargl, J.; Andradas, C.; Brown, A.J.; Irving, A. Minireview: Recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol. Endocrinol. 2011, 25, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauckner, J.E.; Jensen, J.B.; Chen, H.-Y.; Lu, H.-C.; Hille, B.; Mackie, K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. USA 2008, 105, 2699–2704. [Google Scholar] [CrossRef] [Green Version]
- Gendaszewska-Darmach, E.; Drzazga, A. Biological Relevance of Lysophospholipids and Green Solutions for Their Synthesis. Curr. Org. Chem. 2014, 18, 2928–2949. [Google Scholar] [CrossRef]
- Liu, B.; Song, S.; Ruz-Maldonado, I.; Pingitore, A.; Huang, G.C.; Baker, D. GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes. Metab. 2016, 18, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Kaku, K.; Enya, K.; Nakaya, R.; Ohira, T.; Matsuno, R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: A randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes. Metab. 2015, 17, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, J.I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Skelin Klemen, M.; Dolenšek, J.; Slak Rupnik, M.; Stožer, A. The triggering pathway to insulin secretion: Functional similarities and differences between the human and the mouse β cells and their translational relevance. Islets 2017, 9, 109–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClure, K.F.; Darout, E.; Guimarães, C.R.W.; Deninno, M.P.; Mascitti, V.; Munchhof, M.J. Activation of the G-protein-coupled receptor 119: A conformation-base hypothesis for understanding agonist response. J. Med. Chem. 2011, 54, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Frasch, S.C.; Zemski-berry, K.; Murphy, R.C.; Borregaard, N.; Henson, P.M.; Bratton, D.L. Lysophospholipids of Different Classes Mobilize Neutrophil Secretory Vesicles and Induce Redundant Signaling through G2A. J. Immunol. 2007, 178, 6540–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manukyan, L.; Ubhayasekera, S.J.K.A.; Bergquist, J.; Sargsyan, E.; Bergsten, P. Palmitate-Induced Impairments of β-Cell Function Are Linked With Generation of Specific Ceramide Species via Acylation of Sphingosine. Endocrinology 2015, 156, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kren, V.; Walterová, D. Silybin and silymarin—New effects and applications. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2005, 149, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Gazak, R.; Walterova, D.; Kren, V. Silybin and Silymarin—New and Emerging Applications in Medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Barzaghi, N.; Crema, F.; Gatti, G.; Pifferi, G.; Perucca, E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 333–338. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Selim Asker, M.M. Antimicrobical and antivirial impact of novel quercetin-enriched lecithin. J. Food Biochem. 2009, 33, 557–571. [Google Scholar] [CrossRef]
- Villeneuve, P. Lipases in lipophilization reactions. Biotechnol. Adv. 2007, 25, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Pei, K.; Ou, J.; Huang, C.; Ou, S. Derivatives of Ferulic Acid: Structure, Preparation and Biological Activities. Ann. Res. Rev. Biol. 2015, 512–528. [Google Scholar] [CrossRef]
- Balakrishna, M.; Kaki, S.S.; Karuna, M.S.L.; Sarada, S.; Kumar, C.G.; Prasad, R.B.N. Synthesis and in vitro antioxidant and antimicrobial studies of novel structured phosphatidylcholines with phenolic acids. Food Chem. 2017, 221, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Rychlicka, M.; Maciejewska, G.; Niezgoda, N.; Gliszczyńska, A. Production of feruloylated lysophospholipids via a one-step enzymatic interesterification. Food Chem. 2020, 126802. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F. Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J. Nutr. 2004, 134, 3083–3088. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Li, P.; Wang, S.; Prestidge, C.A. Lipophilic Prodrugs of SN38: Synthesis and in vitro Characterization toward Oral Chemotherapy. Mol. Pharm. 2016, 13, 287–294. [Google Scholar] [CrossRef]
- Charman, W.N.; Porter, C.J.H. Lipophilic prodrugs designed for intestinal lymphatic transport. Adv. Drug Deliv. Rev. 1996, 19, 149–169. [Google Scholar] [CrossRef]
- Lambert, D.M. Rationale and Applications of Lipids as Prodrug Carriers. Eur. J. Pharm. Sci. 2000, 11, S15–S27. [Google Scholar] [CrossRef]
- Han, S.; Hu, L.; Quach, T.; Simpson, J.S.; Trevaskis, N.L.; Porter, C.J.H. Profiling the Role of Deacylation-Reacylation in the Lymphatic Transport of a Triglyceride-Mimetic Prodrug. Pharm. Res. 2015, 32, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hu, L.; Gracia Quach, T.; Simpson, J.S.; Edwards, G.A. Lymphatic Transport and Lymphocyte Targeting of a Triglyceride Mimetic Prodrug Is Enhanced in a Large Animal Model: Studies in Greyhound Dogs. Mol. Pharm. 2016, 13, 3351–3361. [Google Scholar] [CrossRef] [PubMed]
- Smani, Y.; Domínguez-Herrera, J.; Ibáñez-Martínez, J.; Pachóna, J. Therapeutic efficacy of lysophosphatidylcholine in severe infections caused by Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 3920–3924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, A.; Duvdevani, R.; Shapiro, I.; Elmann, A.; Finkelstein, E.; Hoffman, A. The oral absorption of phospholipid prodrugs: In vivo and in vitro mechanistic investigation of trafficking of a lecithin-valproic acid conjugate following oral administration. J. Control. Release 2008, 126, 1–9. [Google Scholar] [CrossRef]
- Dahan, A.; Markovic, M.; Epstein, S.; Cohen, N.; Zimmermann, E.M.; Aponick, A. Phospholipid-drug conjugates as a novel oral drug targeting approach for the treatment of inflammatory bowel disease. Eur. J. Pharm. Sci. 2017, 108, 78–85. [Google Scholar] [CrossRef]
- Gadgoli, C.; Mishra, S.H. Antihepatotoxic activity of p-methoxy benzoic acid from Capparis spinosa. J. Ethnopharmacol. 1999, 66, 187–192. [Google Scholar] [CrossRef]
- Narasimhan, B.; Ohlan, S.; Ohlan, R.; Judge, V.; Narang, R. Hansch analysis of veratric acid derivatives as antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 689–700. [Google Scholar] [CrossRef]
- Tao, L.; Wang, S.; Zhao, Y.; Sheng, X.; Wang, A.; Zheng, S. Phenolcarboxylic acids from medicinal herbs exert anticancer effects through disruption of COX-2 activity. Phytomedicine 2014, 21, 1473–1482. [Google Scholar] [CrossRef]
- Saravanakumar, M.; Raja, B. Veratric acid, a phenolic acid attenuates blood pressure and oxidative stress in l-NAME induced hypertensive rats. Eur. J. Pharmacol. 2011, 671, 87–94. [Google Scholar] [CrossRef]
- Drzazga, A.; Ciesielska, A.; Gendaszewska-Darmach, E. Sulfur-and Acyl Chain-Dependent Influence of 2-Methoxy-Lysophosphatidylcholine Analogues on β Pancreatic Cells. Current Topics in Medicinal Chemistry 2015, 15, 2395–2405. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Ahyayauch, H.; Goñi, F.M. The mechanism of detergent solubilization of lipid bilayers. Biophys. J. 2013, 105, 289–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzaki, K.; Handa, T.; Miyajima, K.; Mikura, Y.; Shimizu, H.; Toguchi, H. Quantitative analysis of hemolytic action of lysophosphatidylcholines in vitro: Effect of acyl chain structure. Chem. Pharm. Bull. 1988, 36, 4253–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, R.E.; Fanni, T.; Dennis, E.A. Interfacial Properties and Critical Micelle Concentration of Lysophospholipids. Biochemistry 1989, 28, 5113–5120. [Google Scholar] [CrossRef]
- Zhou, L.C.; Shi, M.J.; Guo, Z.M.; Brisbon, W.; Hoover, R.; Yang, H. Different cytotoxic injuries induced by lysophosphatidylcholine and 7-ketocholesterol in mouse endothelial cells. Endothel. J. Endothel. Cell Res. 2006, 13, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Hill, T.G.; Ashcroft, F.M.; de Baaij, J.H.F. Low extracellular magnesium does not impair glucose-stimulated insulin secretion. PLoS ONE 2019, 14, e0217925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Ohishi, T.; Matsui, T.; Shibasaki, M. Identification of a novel GPR119 agonist, AS1269574, with in vitro and in vivo glucose-stimulated insulin secretion. Biochem. Biophys. Res. Commun. 2010, 400, 437–441. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakamura, H.; Sato, H.; Matsuda, H.; Takada, K.; Tsuji, T. Four Plasma Glucose and Insulin Responses to a 75 g OGTT in Healthy Young Japanese Women. J. Diabetes Res. 2018, 2018, 5742497. [Google Scholar] [CrossRef] [Green Version]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up: A preliminary report. Diabetes Care 2009, 32, 1464–1466. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegała, Ł.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. https://doi.org/10.3390/nu12041173
Drzazga A, Okulus M, Rychlicka M, Biegała Ł, Gliszczyńska A, Gendaszewska-Darmach E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients. 2020; 12(4):1173. https://doi.org/10.3390/nu12041173
Chicago/Turabian StyleDrzazga, Anna, Marta Okulus, Magdalena Rychlicka, Łukasz Biegała, Anna Gliszczyńska, and Edyta Gendaszewska-Darmach. 2020. "Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors" Nutrients 12, no. 4: 1173. https://doi.org/10.3390/nu12041173