Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Permeability of Sesquiterpene Lactones
2.2.1. In Silico Prediction of SLs Permeability
2.2.2. Caco-2 Cell Culture
2.2.3. Evaluation of Caco-2 Cell Viability
2.2.4. Permeability across Caco-2 Monolayer
2.2.5. LC-MS Analysis
2.3. Anti-Inflammatory Potential of Sesquiterpene Lactones
2.3.1. Saccharomyces cerevisiae Strains and Growth Conditions
2.3.2. Cell Viability
2.3.3. β-Galactosidase Assays
2.3.4. Fluorescence Microscopy
2.3.5. Quantitative Real Time PCR
2.3.6. Statistical Analysis
3. Results
3.1. In Silico Prediction of Sls Intestinal Permeability
3.2. In Vitro Permeability of Sls across Caco-2
3.3. Anti-Inflammatory Potential of SLs
3.3.1. Anti-Inflammatory Potential of 11β,13-Dihydrolactucin
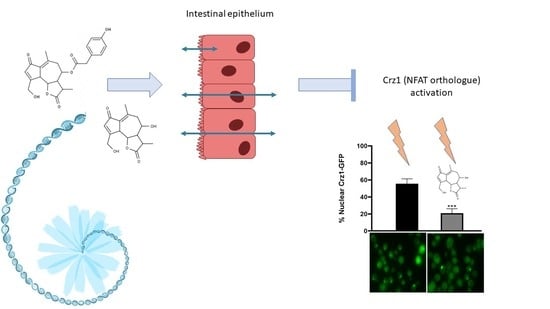
3.3.2. 11β,13-Dihydrolactucin Modulates Crz1 Nuclear Accumulation
3.3.3. 11β,13-Dihydrolactucin Inhibits the Expression of Crz1 Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADME | Absorption, Distribution, Metabolism, Excretion |
| BCRP | Breast cancer resistant protein |
| CDRE | Calcineurin dependent response element |
| COX-2 | Cycloxygenase–2 |
| Crz1 | Calcineurin-responsive zinc finger–1 |
| CSM | Complete supplement mixture |
| DMEM | Dulbecco’s modified eagle medium |
| EIC | Extracted ion chromatogram |
| ESI | Electrospray ionization |
| FBS | Fetal Bovine Serum |
| FKBP12 | FK506-binding protein 12 |
| FT | Fourier transform |
| FWHM | Full width at half maximum |
| GFP | Green Fluorescence Protein |
| GSC2 | Goosecoid–like homeobox protein 2 |
| GSH | Glutathione |
| GST | Glutathione S–transferase |
| HBSS | Hank’s balanced salt solution |
| HPLC | High performance liquid chromatography |
| IκB | Inhibitor of κB |
| IL | Interleukin |
| IT | Ion trap |
| LC | Liquid chromatography |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MDCK | Madin–Darby canine kidney |
| MRP | Multidrug resistance protein |
| MS | Mass spectrometry |
| MU | Miller units |
| m/z | Mass:charge ratio |
| NFAT | Nuclear factor of activated T–cells |
| NF-κB | Nuclear factor–κB |
| OATP | Organic anion transporting polypeptide |
| OD | Optical density |
| ONPG | o–nitrophenyl β–D–galactopyranoside |
| OPLS | Optimal Potential for Liquid Simulations |
| PDA | Photodiode array |
| P-gp | P–glycoprotein |
| PMR1 | Calcium-transporting ATPase 1 |
| PSA | Polar surface area |
| QC | Quality control |
| RT | Retention time |
| SC | Synthetic complete |
| SD | Standard Deviation |
| SEM | Standard Error of the Mean |
| SL | Sesquiterpene lactone |
| TEER | Transepithelial electrical resistance |
| TNF-α | Tumor necrosis factor-alpha |
| UHPLC | Ultrahigh performance liquid chromatography |
| YPD | Yeast extract peptone–dextrose |
| Y-PER | Yeast protein extraction reagent |
References
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid.-Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Gupta, A.K. Applications of inulin and oligofructose in health and nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, C.-X.; Zou, L.; Zhao, Z.-Z.; Zhu, H.; He, Q.-J.; Yang, B.; Gan, L.-S. Terpenoids from Cichorium intybus. Nat. Prod. Commun. 2012, 7, 971–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids Lactones: Benefits to Plants and People. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [Green Version]
- Miglietta, A.; Bozzo, F.; Gabriel, L.; Bocca, C. Microtubule-interfering activity of parthenolide. Chem. Interact. 2004, 149, 165–173. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Kim, D.Y.; Choi, B.Y. Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef] [Green Version]
- Hehner, S.P.; Hofmann, T.G.; Dröge, W.; Schmitz, M.L. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-kappa B by targeting the I kappa B kinase complex. J. Immunol. 1999, 163, 5617–5623. [Google Scholar]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an Anti-Inflammatory Sesquiterpene Lactone from Arnica, Selectively Inhibits Transcription Factor NF-κB. Biol. Chem. 1997, 378, 951–962. [Google Scholar] [CrossRef]
- Pae, H.-O.; Jeong, G.-S.; Woo, W.H.; Rhew, H.Y.; Kim, H.S.; Sohn, D.H.; Kim, Y.-C.; Chung, H. Costunolide inhibits production of tumor necrosis factor-α and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm. Res. 2007, 56, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; Ferracane, R.; Sambo, P.; Santagata, S.; Nicoletto, C.; Fogliano, V. Profiling chicory sesquiterpene lactones by high resolution mass spectrometry. Food Res. Int. 2015, 67, 193–198. [Google Scholar] [CrossRef]
- Wesołowskal, A.; Nikiforuk, A.; Michalska, K.; Kisiel, W.; Chojnacka-Wójcik, E. Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J. Ethnopharmacol. 2006, 107, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, T.A.; Kelley, C.J.; Karchesy, Y.; Laurantos, M.; Nguyen-Dinh, P.; Arefi, A.G. Antimalarial activity of Lactucin and Lactucopicrin: Sesquiterpene lactones isolated from Cichorium intybus L. J. Ethnopharmacol. 2004, 95, 455–457. [Google Scholar] [CrossRef]
- Cavin, C.; Delannoy, M.; Malnoë, A.; Debefve, E.; Touché, A.; Courtois, D.; Schilter, B. Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem. Biophys. Res. Commun. 2005, 327, 742–749. [Google Scholar] [CrossRef]
- Feske, S.; Rao, A.; Hogan, P.G. The Ca2+–calcineurin–NFAT signalling pathway. In Biochemistry of Lipids, Lipoproteins and Membranes, 4th ed.; Elsevier: Amsterdam, The Netherland, 2007; Volume 41, pp. 365–401. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Su, B.; Barndt, R.J.; Chen, H.; Xin, H.; Yan, G.; Chen, L.; Cheng, D.; Heitman, J.; Zhuang, Y.; et al. FKBP12 is the only FK506 binding protein mediating T-cell inhibition by the immunosuppressant FK5061. Transplantation 2002, 73, 1835–1838. [Google Scholar] [CrossRef]
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Klaas, C.A.; Wagner, G.K.; Laufer, S.; Sosa, S.; Della Loggia, R.; Bomme, U.; Pahl, H.L.; Merfort, I. Studies on the Anti-Inflammatory Activity of Phytopharmaceuticals Prepared from Arnica Flowers1. Planta Medica 2002, 68, 385–391. [Google Scholar] [CrossRef]
- Gertsch, J.; Sticher, O.; Schmidt, T.J.; Heilmann, J. Influence of helenanolide-type sesquiterpene lactones on gene transcription profiles in Jurkat T cells and human peripheral blood cells: Anti-inflammatory and cytotoxic effects. Biochem. Pharmacol. 2003, 66, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- QikProp. QikProp User Manual; Schrödinger, LLC: New York, NY, USA, 2012. [Google Scholar]
- Araki, Y.; Wu, H.; Kitagaki, H.; Akao, T.; Takagi, H.; Shimoi, H. Ethanol stress stimulates the Ca2+-mediated calcineurin/Crz1 pathway in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2009, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Santos, C.N.D.; Menezes, R. High-Throughput Yeast-Based Reporter Assay to Identify Compounds with Anti-Inflammatory Potential. Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin, Germany, 2016; Volume 1449, pp. 441–452. [Google Scholar]
- Miller, J.; Miller, J. Experiments in Molecular Genetics, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Menezes, R.; Foito, A.; Jardim, C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexander, C.J.; Outeiro, T.F.; Stewart, D.; et al. Bioprospection of Natural Sources of Polyphenols with Therapeutic Potential for Redox-Related Diseases. Antioxidants 2020, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Mathema, V.B.; Koh, Y.-S.; Thakuri, B.C.; Sillanpää, M. Parthenolide, a Sesquiterpene Lactone, Expresses Multiple Anti-cancer and Anti-inflammatory Activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Jochems, P.G.M.; Garssen, J.; Van Keulen, A.M.; Masereeuw, R.; Jeurink, P.V. Evaluating Human Intestinal Cell Lines for Studying Dietary Protein Absorption. Nutrients 2018, 10, 322. [Google Scholar] [CrossRef] [Green Version]
- Berginc, K.; Žakelj, S.; Levstik, L.; Uršič, D.; Kristl, A. Fluorescein transport properties across artificial lipid membranes, Caco-2 cell monolayers and rat jejunum. Eur. J. Pharm. Biopharm. 2007, 66, 281–285. [Google Scholar] [CrossRef]
- Konishi, Y.; Hagiwara, K.; Shimizu, M. Transepithelial Transport of Fluorescein in Caco-2 Cell Monolayers and Use of Such Transport in In Vitro Evaluation of Phenolic Acid Availability. Biosci. Biotechnol. Biochem. 2002, 66, 2449–2457. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Xu, R.; Peng, Y.; Wang, M.; Li, X. Intestinal Absorption of Isoalantolactone and Alantolactone, Two Sesquiterpene Lactones from Radix Inulae, Using Caco-2 Cells. Eur. J. Drug Metab. Pharmacokinet. 2018, 44, 295–303. [Google Scholar] [CrossRef]
- Koukoulitsa, C.; Geromichalos, G.D.; Skaltsa, H. VolSurf analysis of pharmacokinetic properties for several antifungal sesquiterpene lactones isolated from Greek Centaurea sp. J. Comput. Mol. Des. 2005, 19, 617–623. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 3 November 2020).
- Meyers, J.; Carter, M.; Mok, N.Y.; Brown, N. On the origins of three-dimensionality in drug-like molecules. Futur. Med. Chem. 2016, 8, 1753–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kortagere, S.; Krasowski, M.D.; Ekins, S. The importance of discerning shape in molecular pharmacology. Trends Pharmacol. Sci. 2009, 30, 138–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thormann, U.; Hänggi, R.; Kreuter, M.; Imanidis, G. Membrane transport of nobilin conjugation products and use of the extract of Chamomillae romanae flos influence absorption of nobilin in the Caco-2 model. Eur. J. Pharm. Sci. 2015, 70, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Hayeshi, R.; Hilgendorf, C.; Artursson, P.; Augustijns, P.; Brodin, B.; Dehertogh, P.; Fisher, K.; Fossati, L.; Hovenkamp, E.; Korjamo, T.; et al. Comparison of drug transporter gene expression and functionality in Caco-2 cells from 10 different laboratories. Eur. J. Pharm. Sci. 2008, 35, 383–396. [Google Scholar] [CrossRef]
- Gietl, Y.; Vamvakas, S.; Anders, M.W. Intestinal absorption of S-(pentachlorobutadienyl)glutathione and S-(pentachlorobutadienyl)-L-cysteine, the glutathione and cysteine S-conjugates of hexachlorobuta-1,3-diene. Drug Metab. Dispos. 1991, 19, 703–707. [Google Scholar]
- Schmidt, T.J. Structure-Activity Relationships of Sesquiterpene Lactones. In Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherland, 2006; Volume 33, pp. 309–392. [Google Scholar]
- Liu, Q.; Majdi, M.; Cankar, K.; Goedbloed, M.; Charnikhova, T.; Verstappen, F.W.A.; De Vos, R.C.H.; Beekwilder, J.; Van Der Krol, S.; Bouwmeester, H.J. Reconstitution of the Costunolide Biosynthetic Pathway in Yeast and Nicotiana benthamiana. PLOS ONE 2011, 6, e23255. [Google Scholar] [CrossRef]
- Yildiz, D.; Uslu, C.; Cakir, Y.; Oztas, H. l-Cysteine influx and efflux: A possible role for red blood cells in regulation of redox status of the plasma. Free Radic. Res. 2006, 40, 507–512. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Asp. Med. 2009, 30, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Maher, P.; Lewerenz, J.; Lozano, C.; Torres, J.L. A novel approach to enhancing cellular glutathione levels. J. Neurochem. 2008, 107, 690–700. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Mann, G.E. Redox status in mammalian cells and stem cells during culture in vitro: Critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014, 2, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.I.; Abourashed, E.A.; Walker, L. Transport of Parthenolide across Human Intestinal Cells (Caco-2). Planta Medica 2003, 69, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, B.; Yang, X.-W.; Xu, W.; Zhang, P.; Zou, L.; Zhang, L.-X. Intestinal Permeability of Sesquiterpenes in the Caco-2 Cell Monolayer Model. Planta Medica 2010, 76, 319–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustijns, P.; D’Hulst, A.; Van Daele, J.; Kinget, R. Transport of Artemisinin and Sodium Artesunate in Caco-2 Intestinal Epithelial Cells. J. Pharm. Sci. 1996, 85, 577–579. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Lamoth, F.; Steinbach, W.J. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014, 28, 56–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative Structure−Activity Relationship of Sesquiterpene Lactones as Inhibitors of the Transcription Factor NF-κB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Bagga, A.; Sinha, A.; Sharma, A.; Mehta, A.; Gupta, R.; Gulati, A.; Hari, P.; Dinda, A.K. Calcineurin inhibitor induced nephrotoxicity in steroid resistant nephrotic syndrome. Indian J. Nephrol. 2013, 23, 41. [Google Scholar] [CrossRef]
- Bechstein, W.O. Neurotoxicity of calcineurin inhibitors: Impact and clinical management. Transpl. Int. 2000, 13, 313–326. [Google Scholar] [CrossRef]







| Strain | Genotyping Information | Reference |
|---|---|---|
| YAA3 (BY4742-Crz1-GFP) | his3::CRZ1-GFP-HIS3 | [24] |
| YAA5 (BY4742-CDRE-lacZ) | aur1::AUR1-C-4xCDRE-lacZ | [24] |
| YAA6 (BY4742-crz1_CDRE-lacZ) | aur1::AUR1-C-CDRE-lacZ Δcrz1::KanMX4 | [24] |
| Compound | MW 1 | QP logPo/w 2 | QP logS 3 | PSA 4 | % Human Oral Absorption 5 | QP PCaco-2 6 | QP PMDCK 7 | QP logBB 8 |
|---|---|---|---|---|---|---|---|---|
| Costunolide | 232.32 | 2.67 | −2.97 | 40.49 | 100.00 | 2402.79 | 1276.02 | 0.01 |
| Parthenolide | 248.32 | 1.82 | −1.94 | 52.84 | 100.00 | 2712.92 | 1454.94 | 0.06 |
| Lactucin | 276.29 | 0.10 | −2.08 | 106.60 | 68.61 | 198.54 | 86.21 | −1.19 |
| Lactucopicrin | 410.42 | 1.56 | −3.76 | 141.91 | 68.04 | 61.11 | 24.12 | −2.04 |
| 11β,13-dihydrolactucin | 278.30 | 0.18 | −2.30 | 105.89 | 70.09 | 224.82 | 98.60 | −1.07 |
| 11β,13-dihydrolactucopicrin | 412.44 | 1.79 | −3.70 | 140.56 | 72.56 | 95.54 | 39.21 | −1.68 |
| 15-oxalyl-lactucin | 348.31 | −0.03 | −2.45 | 170.30 | 40.99 | 6.42 | 2.69 | −2.23 |
| 15-oxalyl-lactucopicrin | 482.44 | 1.48 | −4.47 | 204.80 | 39.36 | 1.96 | 0.76 | −3.42 |
| 15-oxalyl-11β,13-dihydrolactucin | 350.32 | 0.08 | −2.65 | 169.25 | 43.07 | 7.61 | 3.23 | −2.07 |
| 15-oxalyl-11β,13-dihydrolactucopicrin | 484.46 | 1.50 | −4.38 | 203.57 | 41.32 | 2.06 | 0.79 | −3.11 |
| Acceptable ranges | 130–725 | −2–6.5 | −6.5–0.5 | 7–200 | − | − | − | −3–1.2 |
| Compound | Concentration of SL (Apical Side) (µM) | % of Decrease in SL Concentration in the Apical Side (t = 4h) | |
|---|---|---|---|
| t = 0 h | t = 4 h | ||
| Costunolide | 13.1 ± 1.8 | <LOD | 100 ** |
| Parthenolide | 11.9 ± 2.0 | <LOQ | 100 ** |
| Lactucin | 8.3 ± 0.7 | 5.9 ± 0.5 | 28.8 *** |
| Lactucopicrin | 12.4 ± 1.3 | <LOQ | 100 ** |
| 11β,13-dihydrolactucin | 9.1 ± 1.3 | 8.3 ± 1.4 | 8.7 |
| 11β,13-dihydrolactucopicrin | 7.0 ± 1.2 | 4.9 ± 0.9 | 29.9 * |
| Compound | RT (min) a | m/zb | Peak Area (Average a.u.) | Apical Concentration t = 4 h (µM Equivalents to Parent SL) |
|---|---|---|---|---|
| Costunolide | 31.30 | 233.153 | - | <LOD |
| Costunolide-Cys | 20.70 | 354.173 | 3.1 × 108 | 95 ± 8.4 |
| Parthenolide | 27.44 | 231.137 | 9.2 × 105 | <LOQ |
| Parthenolide-Cys | 17.14 | 370.168 | 4.6 × 108 | 27 ± 5.9 |
| Lactucin | 13.82 | 277.106 | 6.8 × 107 | 5.9 ± 0.5 |
| Lactucin-Cys | 9.83 | 398.126 | 3.5 × 107 | 2.9 ± 3.6 |
| Lactucopicrin | 22.20 | 411.142 | 1.2 × 106 | <LOQ |
| Lactucopicrin-Cys | 16.10 | 532.162 | 2.4 × 108 | 17.8 ± 2.9 |
| Compound | Concentration (µM) | Calcineurin-Crz1 Inhibition (%) ± SD |
|---|---|---|
| FK506 | 12.5 | 67 ± 13 *** |
| Parthenolide | 12.5 | 20 ± 12 |
| Costunolide | 6.25 | 18 ± 9 |
| Lactucopicrin | 12.5 | 18 ± 9 |
| Lactucin | 12.5 | 20 ± 14 |
| 11β,13-dihydrolactucin | 12.5 | 43 ± 8 *** |
| 11β,13-dihydrolactucopicrin | 12.5 | 26 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.S.; Anastácio, J.D.; Allwood, J.W.; Carregosa, D.; Marques, D.; Sungurtas, J.; McDougall, G.J.; Menezes, R.; Matias, A.A.; Stewart, D.; et al. Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory. Nutrients 2020, 12, 3547. https://doi.org/10.3390/nu12113547
Matos MS, Anastácio JD, Allwood JW, Carregosa D, Marques D, Sungurtas J, McDougall GJ, Menezes R, Matias AA, Stewart D, et al. Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory. Nutrients. 2020; 12(11):3547. https://doi.org/10.3390/nu12113547
Chicago/Turabian StyleMatos, Melanie S., José D. Anastácio, J. William Allwood, Diogo Carregosa, Daniela Marques, Julie Sungurtas, Gordon J. McDougall, Regina Menezes, Ana A. Matias, Derek Stewart, and et al. 2020. "Assessing the Intestinal Permeability and Anti-Inflammatory Potential of Sesquiterpene Lactones from Chicory" Nutrients 12, no. 11: 3547. https://doi.org/10.3390/nu12113547