Variability of Serum Proteins in Chinese and Dutch Human Milk during Lactation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setup and Sample Collection
2.2. Milk Serum Preparation and Concentrations
2.3. Sample Preparation, Dimethyl Labeling, Protein Digestion, and Peptide Analysis
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Level and Type of Milk Serum Proteins in Chinese Human Milk
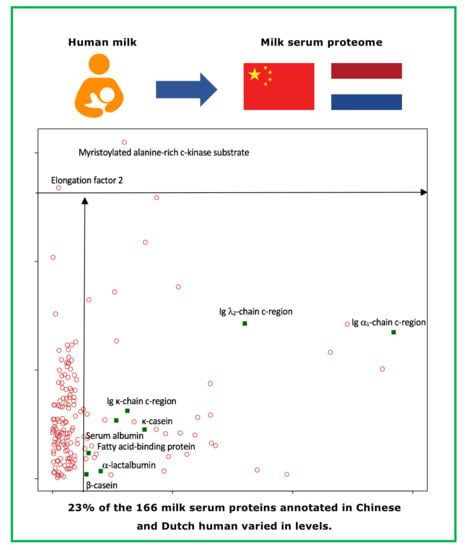
3.2. Comparison of the Chinese and Dutch Milk Serum Proteomes
3.3. Individual Milk Serum Proteins
4. Discussion
4.1. The Level and Type of Serum Proteins in Chinese Human Milk
4.2. The 15 Most Abundant Milk Serum Proteins
4.3. Proteases and Protease Inhibitors
4.4. Comparison of High- and Low- Abundance Serum Proteins in Chinese and Dutch Human Milk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kramer, M.; Kakuma, R. The optimal duration of exclusive breastfeeding: A systematic review. Adv. Exp. Med. Biol. 2004, 554, 63–77. [Google Scholar] [PubMed]
- Liao, Y.; Alvarado, R.; Phinney, B.; Lönnerdal, B. Proteomic characterization of human milk whey proteins during a twelve-month lactation period. J. Proteome Res. 2011, 10, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Ling, P.; Blackburn, G. Review of infant feeding: Key features of breastmilk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.; Morton, J.; Lawrence, R.; Naylor, A.; O’Hare, D.; Schanler, R.; Eidelman, A. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human milk oligosaccharides in colostrum and mature milk of Chinese mothers: Lewis positive secretor subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Lönnerdal, B. Re-evaluation of the whey protein/casein ratio of human milk. Acta Paediatr. 1992, 81, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; de Waard, M.; Verheijen, H.; Boeren, S.; Hageman, J.; van Hooijdonk, T.; Vervoort, J.; van Goudoever, J.; Hettinga, K. Changes over lactation in breast milk serum proteins involved in the maturation of immune and digestive system of the infant. J. Proteomics 2016, 147, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; van Dijk, A.; Hettinga, K. An interactomics overview of the human and bovine proteome over lactation. Proteome Sci. 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, K.; Van Valenberg, H.; De Vries, S.; Boeren, S.; Van Hooijdonk, T.; Van Arendonk, J.; Vervoort, J. The host defense proteome of human and bovine milk. PLoS ONE 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, K.; Reina, F.; Boeren, S.; Zhang, L.; Koppelman, G. Difference in the breast milk proteome between allergic and non-allergic mothers. PLoS ONE 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Weber, D.; Phinney, B.; Smilowitz, J.; Hinde, K.; Lönnerdal, B.; Korf, I.; Lemay, D. Comparative proteomics of human and macaque milk reveals species-specific nutrition during postnatal development. J. Proteome Res. 2015, 14, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Lönnerdal, B. α1-Antitrypsin and antichymotrypsin in human milk: Origin, concentrations, and stability. Am. J. Clin. Nutr. 2002, 76, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, T. Protease inhibitors in human milk. Pediatr. Res. 1982, 16, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.; Murray, N.; Gan, J. Proteolytic systems in milk: Perspectives on the evolutionary function within the mammary gland and the infant. J. Mammary Gland Biol. Neoplasia. 2015, 20, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; O’Flaherty, P.; Fox, P. Indigenous proteolytic enzymes in milk: A brief overview of the present state of knowledge. Int. Dairy J. 2006, 16, 563–572. [Google Scholar] [CrossRef]
- Dingess, K.; de Waard, M.; Boeren, S.; Vervoort, J.; Lambers, T.; van Goudoever, J.; Hettinga, K. Human milk peptides differentiate between the preterm and term infant and across varying lactational stages. Food Funct. 2017, 8, 3769–3782. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.; Guerrero, A.; Khaldi, N.; Borghese, R.; Bhandari, A.; Underwood, M. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J. Nutr. 2014, 144, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Broadhurst, M.; Liu, C.; Gathercole, J.; Cheng, W.; Qi, X. Comparative analysis of human milk and infant formula derived peptides following in vitro digestion. Food Chem. 2017, 221, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Human milk proteins: Key components for the biological activity of human milk. Adv. Exp. Med. Biol. 2004, 554, 11–25. [Google Scholar]
- Lönnerdal, B. Bioactive proteins in human milk: Mechanisms of action. J. Pediatr. 2010, 156, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D. Bioactive components of human milk: Evolution, efficiency and protection. Adv. Exp. Med. Biol. 2001, 501, 3–10. [Google Scholar] [PubMed]
- Spik, G.; Brunet, B.; Mazurier-Dehaine, C.; Fontaine, G.; Montreuil, J. Characterization and properties of the human and bovine lactotransferrins extracted from the faeces of newborn infants. Acta Pædiatr. Scand. 1982, 71, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.; Lönnerdal, B. Persistence of human milk proteins in the breastfed infant. Acta Pædiatr. 1987, 76, 733–740. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Jakaitis, B.; Denning, P. Human breast milk and the gastrointestinal innate immune system. Clin. Perinatol. 2014, 41, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Poulsen, N.; Moller, H.; Stensballe, A.; Larsen, L. Comparative proteomic analysis of casein and whey as prepared by chymosin-induced separation, isoelectric precipitation or ultracentrifugation. J. Dairy Res. 2012, 79, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Boeren, S.; de Vries, S.; van Valenberg, H.; Vervoort, J. Filter-aided sample preparation with dimethyl labeling to identify and quantify milk fat globule membrane proteins. J. Proteomics 2011, 75, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nature 2008, 26, 1367–1372. [Google Scholar]
- Schwanhausser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Sherman, B.; Lempicki, R. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of proteomics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Breckwoldt, J.; Neulen, J.; Keck, C. Lactation. From cellular mechanisms to integration. In Comprehensive Human Physiology; Greger, R., Windhorst, U., Eds.; Springer: Berlin, Germany, 1996; Volume 1, pp. 2365–2373. [Google Scholar]
- Monaco, M.; Donavan, S. Human milk: Nutritional properties. In Nutrition in Pediatrics: Basic Science and Clinical Applications; Walker, W., Watkins, J., Duggan, C., Eds.; BC Decker Inc.: Hamilton, ON, Canada, 2008; Volume 4, pp. 341–353. [Google Scholar]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.; Zhang, G.; Doherty, D.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.; et al. Antimicrobial protein and peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Weber, D.; Xu, W.; Durbin-Johnson, B.; Phinney, B.; Lönnerdal, B. Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. J. Proteome Res. 2017, 16, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B.; Erdmann, P.; Thakkar, S.; Sauser, J.; Destaillats, F. Longitudinal evolution of true protein, amino acids and bioactive proteins in breast milk: A developmental perspective. J. Nutr. Biochem. 2017, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jackson, R.; Khan, S.; Ahuja, J.; Pehrsson, P. Human milk nutrient composition in the United States: Current knowledge, challenges, and research needs. Curr. Dev. Nutr. 2018, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.; Fogleman, A.; Newburg, D.; Allen, J. A longitudinal study of human milk composition in the second year postpartum: Implications for human milk. Matern. Child Nutr. 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]





| Function | Protein Name | BCA Equivalent Values (g/L) | |
|---|---|---|---|
| Chinese | Dutch | ||
| Enzyme | α-lactalbumin | 6.98 | 8.73 |
| Bile salt-activated lipase | 0.29 | 0.19 | |
| Immunity | Lactoferrin | 3.74 | 2.10 |
| Ig α1-chain c-region | 0.91 | 0.71 | |
| Ig λ2-chain c-region | 0.47 | 0.54 | |
| Ig κ-chain c-region | 0.39 | 0.90 | |
| Polymeric immunoglobulin receptor | 0.41 | 0.39 | |
| Clusterin | 0.23 | 0.17 | |
| Osteopontin | 0.17 | 0.19 | |
| β2-microglobulin | 0.16 | 0.16 | |
| Protease inhibitors | α1-antichymotrypsin | 0.11 | 0.08 |
| Transport | β-casein † | 1.17 | 3.91 |
| αS1-casein † | 1.33 | 1.34 | |
| Serum albumin | 0.93 | 1.06 | |
| κ-casein † | 0.23 | 0.29 | |
| Fatty acid-binding protein | 0.07 | 0.13 | |
| Function * | Protein Name | p-Values of Serum Proteins (Chinese Versus Dutch) | |
|---|---|---|---|
| Intercept | Slope | ||
| Cell | Actin | 0.002 * | 0.540 |
| Calreticulin | 0.000 * | 0.620 | |
| Follistatin-related protein 1 | 0.003 * | 0.140 | |
| MARCKS-like protein 1 | 0.004 * | 0.959 | |
| Protein deglycase DJ-1 | 0.000 * | 0.051 | |
| Peroxiredoxin 2 | 0.002 * | 0.233 | |
| Enzyme | 4-trimethylaminobutyraldehyde dehydrogenase | 0.000 * | 0.590 |
| α-lactalbumin | 0.010 * | 0.922 | |
| Fructose-bisphosphate aldolase A | 0.000 * | 0.710 | |
| Isocitrate dehydrogenase 1 | 0.000 * | 0.310 | |
| L-lactate dehydrogenase A | 0.000 * | 0.772 | |
| Nucleoside diphosphate kinase A | 0.000 * | 0.082 | |
| Protein disulfide-isomerase | 0.000 * | 0.685 | |
| Transketolase | 0.023 * | 0.707 | |
| Triosephosphate isomerase | 0.000 * | 0.912 | |
| Tryptophan-tRNA ligase | 0.000 * | 0.131 | |
| UTP-glucose 1-phosphate uridylyltransferase | 0.000 * | 0.630 | |
| Immunity | Complement C4B | 0.000 * | 0.200 |
| Ig α1-chain c-region | 0.000 * | 0.210 | |
| Ig γ3 chain c-region | 0.000 * | 0.190 | |
| Ig κ-chain c-region | 0.001 * | 0.490 | |
| Ig λ2-chain c-region | 0.045 * | 0.640 | |
| Granulins | 0.018 * | 0.800 | |
| Lysozyme C | 0.000 * | 0.937 | |
| Monocyte differentiation antigen CD14 | 0.015 * | 0.770 | |
| Protease inhibitors | Inter-α-trypsin inhibitor heavy chain H2 | 0.000 * | 0.522 |
| Protein synthesis | Elongation factor 2 | 0.547 | 0.050* |
| Signaling | 14-3-3 protein β/α | 0.000 * | 0.372 |
| Transport | Apolipoprotein E | 0.036 * | 0.500 |
| β-casein † | 0.000 * | 0.590 | |
| Fatty acid-binding protein | 0.000 * | 0.790 | |
| Heat shock protein HSP 90-beta | 0.040 * | 0.810 | |
| κ-casein † | 0.038 * | 0.950 | |
| Selenium-binding protein 1 | 0.006 * | 0.536 | |
| Serum albumin | 0.031 * | 0.760 | |
| Transcobalamin 1 | 0.000 * | 0.509 | |
| Other | Myristoylated alanine-rich c-kinase substrate | 0.001 | 0.028 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elwakiel, M.; Boeren, S.; Hageman, J.A.; Szeto, I.M.; Schols, H.A.; Hettinga, K.A. Variability of Serum Proteins in Chinese and Dutch Human Milk during Lactation. Nutrients 2019, 11, 499. https://doi.org/10.3390/nu11030499
Elwakiel M, Boeren S, Hageman JA, Szeto IM, Schols HA, Hettinga KA. Variability of Serum Proteins in Chinese and Dutch Human Milk during Lactation. Nutrients. 2019; 11(3):499. https://doi.org/10.3390/nu11030499
Chicago/Turabian StyleElwakiel, Mohèb, Sjef Boeren, Jos A. Hageman, Ignatius M. Szeto, Henk A. Schols, and Kasper A. Hettinga. 2019. "Variability of Serum Proteins in Chinese and Dutch Human Milk during Lactation" Nutrients 11, no. 3: 499. https://doi.org/10.3390/nu11030499