WUE and CO2 Estimations by Eddy Covariance and Remote Sensing in Different Tropical Biomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Policy and Use License
2.2. Description of Study Area
2.2.1. Cerrado Site
2.2.2. Caatinga Site
2.2.3. Pantanal Site
2.2.4. Amazon Site
2.3. Instrumentation and Data Processing
2.4. Flux Partitioning
2.5. MODIS Data
3. Results
3.1. Meteorological Conditions
3.2. Water and Energy Fluxes
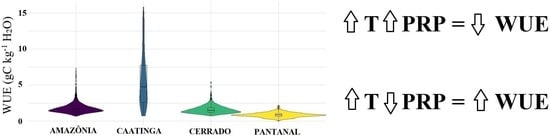
3.3. Carbon Fluxes and WUE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreae, M.O.; Artaxo, P.; Brandão, C.; Carswell, F.E.; Ciccioli, P.; da Costa, A.L.; Culf, A.D.; Esteves, J.L.; Ash, J.H.C.; Grace, J.; et al. Biogeochemical cycling of carbon, water, energy, trace gases, and aerosols in Amazonia: The LBA-EUSTACH experiments. J. Geophys. Res. 2002, 107, 25. [Google Scholar] [CrossRef] [Green Version]
- Araújo, A.C.; Nobre, A.D.; Kruijt, B.; Elbers, J.A.; Dallarosa, R.; Stefani, P.; Randow, C.; von Manzi, A.O.; Culf, A.D.; Gash, J.H.C.; et al. Comparative measurements of carbon dioxide fluxes from two nearby towers in a central Amazonian rainforest: The Manaus LBA site. J. Geophys Res. 2002, 107, 8090. [Google Scholar] [CrossRef] [Green Version]
- Borma, L.D.S.; da Rocha, H.R.; Cabral, O.M.; von Randow, C.; Collicchio, E.; Kurzatkowski, D.; Brugger, P.J.; Freitas, H.; Tannus, R.; Oliveira, L.; et al. Atmosphere and hydrological controls of the evapotranspiration over aflood plain forestin the Bananal Island region, Amazonia. J. Geophys. Res.-Biogeo. 2009, 114, G01003. [Google Scholar] [CrossRef] [Green Version]
- Carswell, F.E.; Costa, A.L.; Palheta, M.; Malhi, Y.; Meir, P.; Costa, J.d.P.R.; Ruivo, M.d.L.; Leal, L.d.S.M.; Costa, J.M.N.; Clement, R.J.; et al. Seasonality in CO2 and H2O flux at an eastern Amazonian rain forest. J. Geophys. Res. 2002, 107, 16. [Google Scholar]
- Rocha, H.R.; Goulden, M.L.; Miller, S.D.; Menton, M.C.; Pinto, L.D.V.O.; De Freitas, H.C.; E Silva Figueira, A.M. Seasonality of water and heat fluxes over a tropical forest in Eastern Amazonia. Ecol. Appl. 2004, 14, 22–32. [Google Scholar] [CrossRef]
- Rocha, H.R.; Manzi, A.O.; Cabral, O.M.; Miller, S.D.; Goulden, M.L.; Saleska, S.R.; Restrepo-Coupe, N.; Wofsy, S.C.; Borma, L.S.; Artaxo, P.; et al. Patterns of water and heat flux across a biome gradient from tropical forest to savanna in Brazil. J. Geophys. Res. 2009, 114, 8. [Google Scholar] [CrossRef]
- Sellers, P.J.; Shuttleworth, W.J.; Dorman, J.L.; Dalcher, A.; Roberts, J.M. Calibrating the Simple Biosphere Model for Amazonian tropical forest using field and remote sensing data. Part I: Average calibration with field data. J. Appl. Meteorol. 1989, 28, 727–759. [Google Scholar]
- Saad, S.I.; da Rocha, H.R.; Silva Dias, M.A.F.; Rosolem, R. Can the deforestation breeze change the rainfall in Amazonia? A case study for the BR-163 highway region. Earth Interact. 2010, 14, 1–25. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Zhou, S.; Yang, X.; Yu, K.; Wang, M.; Liu, S.; Wang, P.; Wu, X.; Wang, X.; et al. Quantifying plant transpiration and canopy conductance using eddy flux data: An underlying water use efficiency method. Agric. For. Meteorol. 2019, 271, 375–384. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; Mutti, P.R.; Ferreira, R.R.; Ramos, T.M.; Marques, T.V.; dos Reis, J.S.; de Lima Vieira, M.M.; Silva, A.C.N.; Marques, A.M.S.; et al. Assessment of SITE for CO2 and Energy Fluxes Simulations in a Seasonally Dry Tropical Forest (Caatinga Ecosystem). Forests 2021, 12, 86. [Google Scholar] [CrossRef]
- Ruhoff, A.L.; Paz, A.R.; Collischonn, W.; Aragao, L.E.O.C.; Rocha, H.R.; Malhi, Y.S. A MODIS-Based Energy Balance to Estimate Evapotranspiration for Clear-Sky Days in Brazilian Tropical Savannas. Remote Sens. 2012, 4, 703–725. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, G.S.; Ruhoff, A.; Cavalcanti, J.R.; Marques, D.M.; Roberti, D.R.; da Rocha, H.R.; Munar, A.M.; Fragoso, C.R.; de Oliveira, M.B.L. Assessing CERES Surface Radiation Components for Tropical and Subtropical Biomes. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2019, 1, 3826–3840. [Google Scholar] [CrossRef]
- Fonseca, L.D.M.; Dalagnol, R.; Malhi, Y.; Rifai, S.W.; Costa, G.B.; Silva, T.S.F.; da Rocha, H.R.; Tavares, I.B.; Borma, L.S. Phenology and Seasonal Ecosystem Productivity in an Amazonian Floodlain Forest. Remote Sens. 2019, 11, 1530. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.A.; Ruhoff, A.L.; Roberti, D.R.; de Arruda Souza, V.; da Rocha, H.R.; Paiva, R.C.D. Assessment of terrestrial water balance using remote sensing data in South America. J. Hydrol. 2019, 575, 131–147. [Google Scholar] [CrossRef]
- Myneni, R.; Knyazikhin, Y.; Park, T. MCD15A3H MODIS/Terra+Aqua Leaf Area Index/FPAR 4-Day L4 Global 500m SIN Grid V006. NASA EOSDIS Land Processes DAAC. Available online: https://lpdaac.usgs.gov/products/mcd15a3hv006/ (accessed on 26 April 2022).
- Laipelt, L.; Ruhoff, A.L.; Fleischmann, A.S.; Kayser, R.H.B.; Kich, E.M.; da Rocha, H.R.; Neale, C.M.U. Assessment of an Automated Calibration of the SEBAL Algorithm to Estimate Dry-Season Surface-Energy Partitioning in a Forest–Savanna Transition in Brazil. Remote Sens. 2020, 12, 1108. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, R.R.; Mutti, P.R.; Mendes, K.R.; Campos, S.; Marques, T.V.; Oliveira, C.P.; Gonçalves, W.; Mota, J.; Difante, G.; Urbano, S.A.; et al. An assessment of the MOD17A2 gross primary production product in the Caatinga biome, Brazil. Int. J. Remote Sens. 2021, 42, 1275–1291. [Google Scholar] [CrossRef]
- Fei, X.H.; Jin, Y.Q.; Zhang, Y.P.; Sha, L.Q.; Liu, Y.T.; Song, Q.H.; Zhou, W.; Liang, N.; Yu, G.; Zhang, L.; et al. Eddy covariance and biometric measurements show that a savanna ecosystem in Southwest China is a carbon sink. Sci. Rep. 2017, 7, 41025. [Google Scholar] [CrossRef] [Green Version]
- Kanniah, K.D.; Beringer, J.; Hutley, L.B. Environmental controls on the spatial variability of savanna productivity in the Northern Territory, Australia. Agric. For. Meteorol. 2011, 151, 1429–1439. [Google Scholar] [CrossRef]
- Quansah, E.; Mauder, M.; Balogun, A.A.; Amekudzi, L.K.; Hingerl, L.; Bliefernicht, J.; Kunstmann, H. Carbon dioxide fluxes from contrasting ecosystems in the Sudanian Savanna in West Africa. Carbon Balance Manag. 2015, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Scheiter, S.; Higgins, S.I.; Beringer, J.; Hutley, L.B. Climate change and long-term fire management impacts on Australian savannas. New Phytol. 2015, 205, 1211–1226. [Google Scholar] [CrossRef]
- Scott, R.L.; Hamerlynck, E.P.; Jenerette, G.D.; Moran, M.S.; Huxman, T.E.; Barron-Gafford, G.A. Carbon dioxide exchange in a semidesert grassland through drought-induced vegetation change. J. Geophys. Res. 2010, 115, G03026. [Google Scholar] [CrossRef]
- Davidson, E.A.; de Araújo, A.C.; Artaxo, P.; Balch, J.K.; Brown, I.F.; Bustamante, M.M.; Coe, M.T.; DeFries, R.S.; Keller, M.; Longo, M.; et al. The Amazon basin in transition. Nature 2012, 481, 321–328. [Google Scholar] [CrossRef] [PubMed]
- WMO. WMO Greenhouse Gas Bulletin (GHG Bulletin)—No.15: The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2018. 2019. Available online: https://library.wmo.int/doc_num.php?explnum_id=10437 (accessed on 27 March 2022).
- Flux Tower BR-Sa1. Available online: https://ameriflux.lbl.gov/sites/siteinfo/BR-Sa1 (accessed on 1 February 2022).
- Flux Tower BR-CST. Available online: https://ameriflux.lbl.gov/sites/siteinfo/BR-CST (accessed on 1 February 2022).
- Flux Tower BR-Npw. Available online: https://ameriflux.lbl.gov/sites/siteinfo/BR-Npw (accessed on 1 February 2022).
- Alcântara, L.R.P.; Coutinho, A.P.; dos Santos Neto, S.M.; Carvalho de Gusmão da Cunha Rabelo, A.E.; Antonino, A.C.D. Modeling of the Hydrological Processes in Caatinga and Pasture Areas in the Brazilian Semi-Arid. Water 2021, 13, 1877. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; Zanella de Arruda, P.H.; Vourlitis, G.L.; Lathuilliere, M.J.; de Nogueira, S.J.; Couto, E.G.; Johnson, M.S. Radiative forcing of methane fluxes offsets net carbon dioxide uptake for a tropical flooded forest. Glob. Change Biol. 2019, 25, 1967–1981. [Google Scholar] [CrossRef]
- Da Silva, J.B.; Valle Junior, L.C.G.; Faria, T.O.; Marques, J.B.; Dalmagro, H.J.; Nogueira, J.S.; Vourlitis, G.L.; Rodrigues, T.R. Temporal Variability in Evapotranspiration and Energy Partitioning over a Seasonally Flooded Scrub Forest of the Brazilian Pantanal. Agric. For. Meteorol. 2021, 308, 108559. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Fantin-Cruz, I.; Girard, P.; Zeilhofer, P.; Collischonn, W.; da Cunha, C.N. Unidades fitofisionômicas em mesoescala no Pantanal Norte e suas relações com a geomorfologia. Biota Neotropica 2010, 10, 31–38. [Google Scholar] [CrossRef]
- Johnson, M.S.; Couto, E.G.; Pinto, O.B.; Milesi, J.; Santos Amorim, R.S.; Messias, I.A.M.; Biudes, M.S. Soil CO2 Dynamics in a Tree Island Soil of the Pantanal: The Role of Soil Water Potential. PLoS ONE 2013, 8, e64874. [Google Scholar] [CrossRef]
- Rodrigues, T.R.; Curado, L.F.A.; Novais, J.W.Z.; de Oliveira, A.G.; de Paulo, S.R.; Nogueira, D.S. Distribuição sazonal dos componentes do balanço de energia no norte do Pantanal. Rev. Ciências Agro-Ambient. 2011, 9, 165–175. [Google Scholar]
- Biudes, M.S.; Machado, N.G.; Danelichen, V.H.d.M.; Souza, M.C.; Vourlitis, G.L.; Nogueira, J.d.S. Ground and remote sensing-based measurements of leaf area index in a transitional forest and seasonal flooded forest in Brazil. Int. J. Biometeorol. 2014, 58, 1181–1193. [Google Scholar] [CrossRef]
- Couto, E.; Klinger, P.; Jacomine, T.; Nunes Da Cunha, C.; Vechiatto, A. Guide of technique excursion of the XIV RBMCSA. In XIV Reunião Brasileira de Manejo e Conservação do solo e da água; UFMT: Cuiabá, Brazil, 2016; 68p. [Google Scholar]
- Vourlitis, G.L.; de Almeida Lobo, F.; Lawrence, S.; Holt, K.; Zappia, A.; Pinto, O.B.; de Souza Nogueira, J. Nutrient resorption in tropical savanna forests and woodlands of central Brazil. Plant Ecol. 2014, 215, 963–975. [Google Scholar] [CrossRef]
- Prado, M.J.D. Intercâmbio Gasoso e Relações Hídricas de Duas Espécies de Combretum no Pantanal Mato-Grossense. Master’s Thesis, Federal University of Mato Grosso, Campo Grande, Brazil, 2015; p. 56. [Google Scholar]
- Santos, S.A.; da Cunha, C.N.; Tomás, W.; de Abreu, U.G.P.; Arieira, J. Plantas Invasoras no Pantanal: Como Entender o Problema e Soluções de Manejo por Meio de Diagnóstico Participativo; Boletim de Pesquisa e Desenvolvimento 66; Embrapa Pantanal: Corumbá, Brazil, 2006; p. 45. [Google Scholar]
- Pott, V.J.; Pott, A. Plantas do Pantanal Corumbá: Embrapa—Centro de Pesquisa Agropecuária do Pantanal; Embrapa Pantanal: Corumbá, Brazil, 1994; 320p. [Google Scholar]
- Junk, W.J.; da Cunha, C.N.; Wantzen, K.M.; Petermann, P.; Strüssmann, C.; Marques, M.I.; Adis, J. Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquat. Sci. 2006, 68, 278–309. [Google Scholar] [CrossRef]
- Rice, A.H.; Pyle, E.H.; Saleska, S.R.; Hutyra, L.; Palace, M.; Keller, M.; de Camargo, P.B.; Portilho, K.; Marques, D.F.; Wofsy, S.C. Carbon balance and vegetation dynamics in an old-growth Amazonian forest. Ecol. Appl. 2004, 14, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.B. Abolishing virginity. J. Trop. Ecol. 1996, 12, 735–739. [Google Scholar] [CrossRef]
- Stark, S.; Leitold, V.; Wu, J.; Hunter, M.; Castilho, C.; Costa, F.; McMahon, S.; Parker, G.; Shimabukuro, M.; Lefsky, M.; et al. Amazon forest carbon dynamics predicted by profiles of canopy leaf area and light environment. Ecol. Lett. 2012, 15, 1406–1414. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.C.; Rêgo Mendes, K.; Santos e Silva, C.M.; Torres Rodrigues, D.; Brito Costa, G.; Thainara Corrêa da Silva, D.; Rodrigues Mutti, P.; Rodrigues Ferreira, R.; Guedes Bezerra, B. Energy Balance, CO2 Balance, and Meteorological Aspects of Desertification Hotspots in Northeast Brazil. Water 2021, 13, 2962. [Google Scholar] [CrossRef]
- Hutyra, L.R.; Munger, J.W.; Saleska, S.R.; Gottlieb, E.; Daube, B.C.; Dunn, A.L.; Amaral, D.F.; de Camargo, P.B.; Wofsy, S.C. Seasonal controls on the exchange of carbon and water in an Amazonian rain forest. J. Geophys. Res. 2007, 112, 1–16. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Giulmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef] [Green Version]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.D.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Change Biol. 2010, 16, 187–208. [Google Scholar] [CrossRef] [Green Version]
- Monteith, J.L. Solar Radiation and Productivity in Tropical Ecosystems. J. Appl. Ecol. 1972, 9, 747. [Google Scholar] [CrossRef] [Green Version]
- Myneni, R.; Knyazikhin, Y.; Park, T. 15. MOD15A2H MODIS/Terra Leaf Area Index/FPAR 8-Day L4 Global 500 m SIN Grid V006 [Data Set]. NASA EOSDIS Land Processes DAAC. Available online: https://catalog.data.gov/dataset/modis-terraaqua-leaf-area-index-fpar-4-day-l4-global-500m-sin-grid-v006 (accessed on 26 April 2022).
- Rienecker, M.M.; Suarez, M.J.; Gelaro, R.; Todling, R.; Bacmeister, J.; Liu, E.; Woollen, J. 15. MERRA: NASA’s Modern-Era Retrospective Analysis for Research and Applications. J. Clim. 2011, 24, 3624–3648. [Google Scholar] [CrossRef]
- Pei, Y.; Dong, J.; Zhang, Y.; Yang, J.; Zhang, Y.; Jiang, C.; Xiao, X. Performance of Four State-of-the-art GPP Products (VPM, MOD17, BESS and PML) for Grasslands in Drought Years. Ecol. Inform. 2020, 56, 101052. [Google Scholar] [CrossRef]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.; Reeves, M.; Hashimoto, H. A Ontinuous Satellite–derived Measure of Global Terrestrial Primary Production. Bioscience 2004, 54, 547–560. [Google Scholar] [CrossRef]
- Friedl, M.A.; Sulla-Menashe, D.; Tan, B.; Schneider, A.; Ramankutty, N.; Sibley, A.; Huang, X. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens. Environ. 2010, 114, 168–182. [Google Scholar] [CrossRef]
- Zhu, X.; Pei, Y.; Zheng, Z.; Dong, J.; Zhang, Y.; Wang, J.; Chen, L.; Doughty, R.; Zhang, G.; Xiao, X. Underestimates of Grassland Gross Primary Production in MODIS Standard Products. Remote Sens. 2018, 10, 1771. [Google Scholar] [CrossRef] [Green Version]
- Danelichen, V.H.M.; Biudes, M.S.; Velasque, M.C.S.; Machado, N.G.; Gomes, R.S.R.; Vourlitis, G.L.; Nogueira, J.S. Estimating of gross primary production in an Amazon-Cerrado transitional forest using MODIS and Landsat imagery. An. Acad. Bras. Cienc. 2015, 87, 1545–1564. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Dong, J.; Liu, J.; Huang, M.; Li, G.; Running, S.W.; Smith, W.K.; Harris, W.; Saigusa, N.; Kondo, H.; et al. Comparison of gross primary productivity derived from GIMMS NDVI3g, GIMMS, and MODIS in southeast Asia. Remote Sens. 2014, 6, 2108–2133. [Google Scholar] [CrossRef] [Green Version]
- Kuglitsch, F.G.; Reichstein, M.; Beer, C.; Carrara, A.; Ceulemans, R.; Granier, A.; Janssens, I.A.; Köstner, B.; Lindroth, A.; Loustau, D.; et al. Characterisation of ecosystem water-use efficiency of European forests from eddy-covariance measurements. Biogeosciences Discuss. 2008, 5, 4481–4519. [Google Scholar] [CrossRef] [Green Version]
- Berbigier Gier, P.; Bonnefond, J.-M.; Mellmann, P. CO2 and water vapour fluxes for 2 years above Euroflux forest site. Agric. Forest Meterol. 2001, 108, 183–197. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Li, R.; Long, F.; Zhang, L.; Zhang, Q.; Li, J. Water-use efficiency of an old-growth forest in lower subtropical China. Sci. Rep. 2017, 7, 42761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.; Li, H.; Desai, A.R.; Nagy, Z.; Luo, J.; Kolb, T.E.; Olioso, A.; Xu, X.; Yao, L.; Kutsch, W.; et al. How is water-use efficiency of terrestrial ecosystems distributed and changing on Earth? Sci. Rep. 2014, 4, 7483. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.R.; Zhang, L.M.; Sun, X.M.; Fu, Y.L.; Wen, X.F.; Wang, Q.F.; Li, S.G.; Ren, C.Y.; Song, X.I.A.; Liu, Y.F.; et al. Environmental controls over carbon exchange of three forest ecosystems in eastern China. Glob. Change Biol. 2008, 14, 2555–2571. [Google Scholar] [CrossRef]
- Rodrigues, A.; Pita, G.; Mateus, J.; Kurz-Besson, C.; Casquilho, M.; Cerasoli, S.; Gomes, A.; Pereira, J. Eight years of continuous carbon fluxes measurements in a Portuguese eucalypt stand under two main events: Drought and felling. Agric. Forest Meterol. 2011, 151, 493–507. [Google Scholar] [CrossRef]
- Law, B.E.; Williams, M.; Anthoni, P.M.; Baldocchi, D.D.; Unsworth, M.H. Measuring and modelling seasonal variation of carbon dioxide and water vapour exchange of a Pinus ponderosa forest subject to soil water deficit. Glob. Change Biol. 2000, 6, 613–630. [Google Scholar] [CrossRef]
- Krishnan, P.; Black, T.A.; Grant, N.J.; Barr, A.G.; Hogg, E.T.H.; Jassal, R.S.; Morgenstern, K. Impact of changing soil moisture distribution on net ecosystem productivity of a boreal aspen forest during and following drought. Agric. Forest Meterol. 2006, 139, 208–223. [Google Scholar] [CrossRef]
- Ponton, S.; Flanagan, L.B.; Alstad, K.P.; Johnson, B.G.; Morgenstern, K.A.I.; Kljun, N.; Black, T.A.; Barr, A.G. Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob. Change Biol. 2006, 12, 294–310. [Google Scholar] [CrossRef]
- Liang, S.; Li, X.; Wang, J. Estimate of vegetation production of terrestrial ecosystem. In Advanced Remote Sensing: Terrestrial Information Extraction and Applications; Academic Press: Oxford, UK, 2020; pp. 581–620. [Google Scholar]
- Costa, M.H.; Biajoli, M.C.; Sanches, L.; Malhado, A.C.M.; Hutyra, L.R.; da Rocha, H.R.; Aguiar, R.G.; de Araújo, A.C. Atmospheric versus vegetation controls of Amazonian tropical rain forest evapotranspiration: Are the wet and seasonally dry rain forests any different? J. Geophys. Res. 2010, 115, 9. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; de Lobo, F.A.; Vourlitis, G.L.; Dalmolin, Â.C.; Antunes, M.Z.; Ortíz, C.E.R.; de Nogueira, J.S. Photosynthetic parameters of two invasive tree species of the Brazilian Pantanal in response to seasonal flooding. Photosynthetica 2013, 51, 281–294. [Google Scholar] [CrossRef]
- Dalmagro, H.J.; Lathuillière, M.J.; Vourlitis, G.L.; Campos, R.C.; Pinto, O.B.; Johnson, M.S.; Ortíz, C.E.; Lobo, F.D.A.; Couto, E.G. Physiological responses to extreme hydrological events in the Pantanal wetland: Heterogeneity of a plant community containing super-dominant species. J. Veg. Sci. 2016, 27, 568–577. [Google Scholar] [CrossRef]
- Sanches, L.; Vourlitis, G.L.; de Carvalho Alves, M.; Pinto-Júnior, O.B.; de Souza Nogueira, J. Seasonal patterns of evapotranspiration for a Vochysia divergens forest in the Brazilian Pantanal. Wetlands 2011, 31, 1215–1225. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; de Almeida Lobo, F.; Biudes, M.S.; Rodríguez Ortíz, C.E.; de Souza Nogueira, J. Spatial Variations in Soil Chemistry and Organic Matter Content across a Vochysia Divergens Invasion Front in the Brazilian Pantanal. Soil Sci. Soc. Am. J. 2011, 75, 1554–1561. [Google Scholar] [CrossRef]
- Sjöström, M.; Ardö, J.; Arneth, A.; Boulain, N.; Cappelaere, B.; Eklundh, L.; de Grandcourt, A.; Kutsch, W.L.; Merbold, L.; Nouvellon, Y.; et al. Exploring the potential of MODIS EVI for modeling gross primary production across African ecosystems. Remote Sens. Env. 2011, 115, 1081–1089. [Google Scholar] [CrossRef]
- Singh, N.; Patel, N.R.; Bhattacharya, B.K.; Soni, P.; Parida, B.R.; Parihar, J.S. Analyzing the dynamics and inter-linkages of carbon and water fluxes in subtropical pine (Pinus roxburghii) ecosystem. Agric. Forest Meterol. 2014, 197, 206–218. [Google Scholar] [CrossRef]
- Ito, A.; Inatomi, M. Water-use efficiency of the terrestrial biosphere: A model analysis focusing on interactions between the global carbon and water cycles. J. Hydrometeorol. 2012, 13, 681–694. [Google Scholar] [CrossRef]
- Xue, B.L.; Guo, Q.H.; Otto, A.; Xiao, J.F.; Tao, S.L.; Li, L. Global patterns, trends, and drivers of water use efficiency from 2000 to 2013. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—past, present, and future. Theor. Appl. Climatol. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; da Silva, L.L.; Mutti, P.R.; Ferreira, R.R.; Medeiros, S.S.; Perez-Marin, A.M.; Marques, T.V.; Ramos, T.M.; de Lima Vieira, M.M.; et al. Seasonal variation in net ecosystem CO2 exchange of a Brazilian seasonally dry tropical forest. Sci. Rep. 2020, 10, 9454. [Google Scholar] [CrossRef]
- Campos, S.; Mendes, K.R.; da Silva, L.L.; Mutti, P.R.; Medeiros, S.S.; Amorim, L.B.; dos Santos, C.A.C.; Perez-Marin, A.M.; Ramos, T.M.; Marques, T.V.; et al. Closure and partitioning of the energy balance in a preserved area of Brazilian seasonally dry tropical forest. Agric. For. Meteorol. 2019, 271, 398–412. [Google Scholar] [CrossRef]
- Marques, T.V.; Mendes, K.; Mutti, P.; Medeiros, S.; Silva, L.; Perez-Marin, A.M.; Campos, S.; Lúcio, P.S.; Lima, K.; Reis, J.; et al. Environmental and biophysical controls of evapotranspiration from Seasonally Dry Tropical Forests (Caatinga) in the Brazilian Semiarid. Agric. For. Meteorol. 2020, 287, 107957. [Google Scholar] [CrossRef]
- Mendes, K.R.; Batista-Silva, W.; Dias-Pereira, J.; Pereira, M.P.; Souza, E.V.; Serrão, J.E.; Granja, J.A.; Pereira, E.C.; Gallacher, D.J.; Mutti, P.R.; et al. Leaf plasticity across wet and dry seasons in Croton blanchetianus (Euphorbiaceae) at a tropical dry forest. Sci. Rep. 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]











| Forest Type | Slope | R2 | Reference |
|---|---|---|---|
| Alpine grassland | 1.30 | 0.50 | Zhu et al. [55] |
| Alpine grassland | 0.58 | 0.17 | Zhu et al. |
| Dry tropical forest | 0.24 | 0.27 | This study |
| Floodplain forest | 5.39 | 0.01 | This study |
| Primary forest | 5.34 | 0.02 | This study |
| Semi-deciduous forest | 0.49 | 0.32 | Danelichen et al. [56] |
| Temperate grassland | 1.59 | 0.70 | Zhu et al., 2018 |
| Temperate grassland | 0.50 | 0.40 | Zhu et al., 2018 |
| Tropical grassland | 0.89 | 0.53 | Zhu et al., 2018 |
| Tropical peatland | 0.23 | 0.16 | Wang et al. [57] |
| Tropical grassland | 0.91 | 0.63 | Zhu et al., 2018 |
| Tropical grassland | 0.89 | 0.53 | Zhu et al., 2018 |
| Wetland | 2.40 | 0.31 | This study |
| Forest Types | WUE (g C kg−1H2O) | References |
|---|---|---|
| Wetland | 0.95 | This study |
| Boreal treeless wetland | 1.2 | Kuglitsch et al. [58] |
| Floodplain forest | 1.61 | This study |
| Maritime pine | 1.69 | Berbigier et al. [59] |
| Primary forest | 1.82 | This study |
| Deciduous broadleaf forest | 1.87 | Wang et al. |
| Old-growth forest | 1.83 | Liu et al. [60] |
| Evergreen broadleaf forest | 2.35 | Tang et al. [61] |
| Conifer plantation forest | 2.53 | Yu et al. [62] |
| Deciduous broadleaf forest | 2.57 | Yu et al. |
| Eucalypt plantation | 2.87 | Rodrigues et al. [63] |
| Ponderosa pine | 2.97 | Law et al. [64] |
| Evergreen broadleaf forest | 3.13 | Tang et al. |
| Boreal aspen | 3.70 | Krishnan et al. [65] |
| Temperate broad-leaved deciduous | 5.0 | Kuglitsch et al. |
| Douglas-fir | 5.40 | Ponton et al. [66] |
| Dry tropical forest | 5.79 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, G.B.; Santos e Silva, C.M.; Mendes, K.R.; dos Santos, J.G.M.; Neves, T.T.A.T.; Silva, A.S.; Rodrigues, T.R.; Silva, J.B.; Dalmagro, H.J.; Mutti, P.R.; et al. WUE and CO2 Estimations by Eddy Covariance and Remote Sensing in Different Tropical Biomes. Remote Sens. 2022, 14, 3241. https://doi.org/10.3390/rs14143241
Costa GB, Santos e Silva CM, Mendes KR, dos Santos JGM, Neves TTAT, Silva AS, Rodrigues TR, Silva JB, Dalmagro HJ, Mutti PR, et al. WUE and CO2 Estimations by Eddy Covariance and Remote Sensing in Different Tropical Biomes. Remote Sensing. 2022; 14(14):3241. https://doi.org/10.3390/rs14143241
Chicago/Turabian StyleCosta, Gabriel B., Cláudio M. Santos e Silva, Keila R. Mendes, José G. M. dos Santos, Theomar T. A. T. Neves, Alex S. Silva, Thiago R. Rodrigues, Jonh B. Silva, Higo J. Dalmagro, Pedro R. Mutti, and et al. 2022. "WUE and CO2 Estimations by Eddy Covariance and Remote Sensing in Different Tropical Biomes" Remote Sensing 14, no. 14: 3241. https://doi.org/10.3390/rs14143241