Strigolactone-Mediated Mitigation of Negative Effects of Salinity Stress in Solanum lycopersicum through Reducing the Oxidative Damage
Abstract
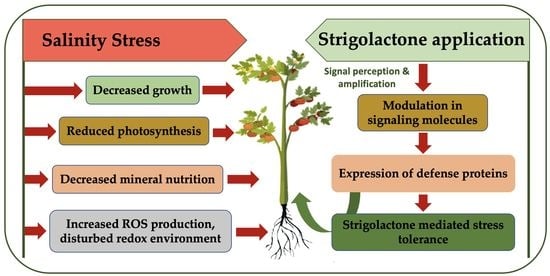
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Source of SLs, Growth Conditions, and Salinity Treatment
2.2. Growth Indices
2.3. Chlorophyll Index and Photosynthetic Attributes
2.4. Antioxidant Enzyme Activity and Proline Content
2.5. Lipid Peroxidation, Hydrogen Peroxide (H2O2), and Protein Content
2.6. Statistical Analysis
3. Results
3.1. Growth Indices
3.2. Chlorophyll Index and Photosynthetic Attributes
3.3. Antioxidant Enzymes Activity and Proline Content
3.4. MDA, H2O2, and Protein Content
4. Discussion

5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Zhu, J.; Khan, K. Effects of genotype and environment on glutenin polymers and bread making quality. Cereal Chem. 2001, 78, 125–130. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Wang, L.; Si, L.; Zheng, S.; Yang, Y.; Yang, H.; Tian, S. Wheat TabZIP8, 9, 13 participate in ABA biosynthesis in NaCl-stressed roots regulated by TaCDPK9-1. Plant Physiol. Biochem. 2020, 151, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, L.; Chen, Y.; Wang, S.; Fang, Y.; Zhang, X.; Wu, Y.; Xue, D. Genome-wide identification of the SOD gene family and expression analysis under drought and salt stress in barley. Plant Growth Regul. 2021, 94, 49–60. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Botany 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S. Salinity and programmed cell death: Unravelling mechanisms for ion specific signalling. J. Exp. Botany 2009, 60, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Alam, P.; Balawi, T.A.; Faizan, M. Salicylic Acid’s Impact on Growth, Photosynthesis, and Antioxidant Enzyme Activity of Triticum aestivum When Exposed to Salt. Molecules 2023, 28, 100. [Google Scholar] [CrossRef]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, N. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nut. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Abiotic stress, generation of reactive oxygen species, and their consequences: An overview. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 23–50. [Google Scholar]
- Faizan, M.; Faraz, A.; Hayat, S. Dose Dependent Response of Epibrassinolide on the Growth, Photosynthesis and Antioxidant System of Tomato Plants. Ind. Hort. J. 2018, 8, 68–76. [Google Scholar]
- Smith, S.M. Q&A: What are strigolactones and why are they important to plants and soil microbes? BMC Biol. 2014, 12, 1–7. [Google Scholar]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Sami, F.; Siddiqui, H.; Yusuf, M.; Gruszka, D.; Hayat, S. Role of strigolactones: Signalling and crosstalk with other phytohormones. Open Life Sci. 2020, 15, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Faizan, M.; Cheng, S.H.; Tonny, S.H.; Robab, M.I. Specific roles of strigolactones in plant physiology and remediation of heavy metals from contaminated soil. Plant Physiol. Biochem. 2022, 192, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Liu, Y.; Yu, Y.; Li, H.; Wang, X.; Pang, Y.; Cao, H.; Sun, Q. Molecular and physiological mechanisms of strigolactones-mediated drought stress in crab apple (Malus hupehensis Rehd.) seedlings. Sci. Horti. 2023, 311, 111800. [Google Scholar] [CrossRef]
- Ahsan, M.; Zulfiqar, H.; Farooq, M.A.; Ali, S.; Tufail, A.; Kanwal, S.; Shaheen, M.R.; Sajid, M.; Gul, H.; Jamal, A.; et al. Strigolactone (GR24) Application Positively Regulates Photosynthetic Attributes, Stress-Related Metabolites and Antioxidant Enzymatic Activities of Ornamental Sunflower (Helianthus annuus cv. Vincent’s Choice) under Salinity Stress. Agriculture 2023, 13, 50. [Google Scholar] [CrossRef]
- Kleman, J.; Matusova, R. Strigolactones: Current research progress in the response of plants to abiotic stress. Biologia 2023, 78, 307–318. [Google Scholar] [CrossRef]
- Soliman, S.; Wang, Y.; Han, Z.H.; Pervaiz, T.; El-kereamy, A. Strigolactones in Plants and Their Interaction with the Ecological Microbiome in Response to Abiotic Stress. Plants 2022, 11, 3499. [Google Scholar] [CrossRef]
- Bhoi, A.; Yadu, B.; Chandra, J.; Keshavkant, S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta 2021, 254, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.W.; Hu, Y.; Beyyoudh, L.; Yildiz Dasgan, H.; Kunert, K.; Beveridge, C.A.; Foyer, C.H. Strigolactones positively regulate chilling tolerance in pea and in Arabidopsis. Plant Cell Environ. 2018, 41, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Visentin, I.; Vitali, M.; Ferrero, M.; Zhang, Y.; Ruyter-Spira, C.; Novák, O.; Strnad, M.; Lovisolo, C.; Schubert, A.; Cardinale, F. Low levels of strigolactones in roots as a component of the systemic signal of drought stress in tomato. New Phytol. 2016, 212, 954–963. [Google Scholar] [CrossRef]
- Ha, C.V.; Leyva-González, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [Green Version]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [Green Version]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Koltai, H. Strigolactones activate different hormonal pathways for regulation of root development in response to phosphate growth conditions. Ann. Bot. 2013, 112, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Zhang, Y.; Li, C.; Liu, Z.; Yang, N.; Pan, L.; Wu, J.; Wang, J.; Yang, J.; Lv, Y. Strigolactone-triggered stomatal closure requires hydrogen peroxide synthesis and nitric oxide production in an abscisic acid-independent manner. New Phytol. 2018, 217, 290–304. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agricul. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernández, J.M.; Bolarin, M.C.; Egea, I. Recovering tomato landraces to simultaneously improve fruit yield and nutritional quality against salt stress. Front. Plant Sci. 2018, 9, 1778. [Google Scholar] [CrossRef] [Green Version]
- Faizan, M.; Hayat, S. Effect of foliar spray of ZnO-NPs on the physiological parameters efficiency and antioxidant systems of Lycopersicum esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Sci. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Benefits of brassinosteroid crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kacar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Horti. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.A.; Cui, X.; Wang, K.F.; Wang, Y.; He, Y.H. Phytohormones regulate the abiotic stress: An overview of physiological, biochemical, and molecular responses in horticultural crops. Front. Plant Sci. 2023, 13, 1095363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Xiao, L. Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed. Res. Int. 2016, 2016, 8231873. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, Y.; Shahbaz, M. GR24 Triggered Variations in Morpho-Physiological Attributes of Sunflower (Helianthus annuus) under Salinity. Int. J. Agri. Biol. 2019, 21, 34–40. [Google Scholar]
- Ruyter-Spira, C.; Kohlen, W.; Charnikhova, T.; van Zeijl, A.; van Bezouwen, L.; de Ruijter, N.; Cardoso, C.; Lopez-Raez, J.A.; Matusova, R.; Bours, R.; et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011, 155, 721–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koren, D.; Resnick, N.; Gati, E.M.; Belausov, E.; Weininger, S.; Kapulnik, Y.; Koltai, H. Strigolactone signaling in the endodermis is sufficient to restore root responses and involves SHORT HYPOCOTYL 2 (SHY2) activity. New Phytol. 2013, 198, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Steduto, P.; Albrizio, R.; Giorio, P.; Sorrentino, G. Gas exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000, 44, 243–255. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T.G. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Horti. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Ma, N.; Hu, C.; Wan, L.; Hu, Q.; Xiong, J.L.; Zhang, C.L. Strigolactones Improve Plant Growth, Photosynthesis, and Alleviate Oxidative Stress under Salinity in Rapeseed (Brassica napus L.) by Regulating Gene Expression. Front. Plant Sci. 2017, 8, 1671. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. 5-aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef] [PubMed]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mathew, L.; Burritt, D.J.; McLachlan, A.; Pathirana, R. Combined pre-treatments enhance antioxidant metabolism and improve survival of cryopreserved kiwifruit shoot tips. Plant Cell Tiss. Organ Cult. 2019, 138, 193–205. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Su, Q.; Jiang, H.; Cui, J.; He, X.; Wu, Z.; Zhang, Z.; Liu, J.; Zhao, Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 6183. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M. Strigolactones as part of the plant defence system. Trends Plant Sci. 2016, 21, 900–903. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ma, C.; Zhang, L.; Su, M.; Wang, J.; Zheng, S.; Zhang, T.G. GR24-mediated enhancement of salt tolerance and roles of H2O2 and Ca2+ in regulating this enhancement in cucumber. J. Plant Physiol. 2022, 270, 153640. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Strigolactones: Multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol. Plant 2018, 40, 86. [Google Scholar] [CrossRef]
- Athar, H.U.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt stress proteins in plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Taghvaei, M.; Nasrolahizadehi, A.; Mastinu, A. Effect of Light, Temperature, Salinity, and Halopriming on Seed Germination and Seedling Growth of Hibiscus sabdariffa under Salinity Stress. Agronomy 2022, 12, 2491. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Faizan, M.; Tonny, S.H.; Rajput, V.D.; Minkina, T.; Alatar, A.A.; Pathirana, R. Strigolactone-Mediated Mitigation of Negative Effects of Salinity Stress in Solanum lycopersicum through Reducing the Oxidative Damage. Sustainability 2023, 15, 5805. https://doi.org/10.3390/su15075805
Faisal M, Faizan M, Tonny SH, Rajput VD, Minkina T, Alatar AA, Pathirana R. Strigolactone-Mediated Mitigation of Negative Effects of Salinity Stress in Solanum lycopersicum through Reducing the Oxidative Damage. Sustainability. 2023; 15(7):5805. https://doi.org/10.3390/su15075805
Chicago/Turabian StyleFaisal, Mohammad, Mohammad Faizan, Sadia Haque Tonny, Vishnu D. Rajput, Tatiana Minkina, Abdulrahman A. Alatar, and Ranjith Pathirana. 2023. "Strigolactone-Mediated Mitigation of Negative Effects of Salinity Stress in Solanum lycopersicum through Reducing the Oxidative Damage" Sustainability 15, no. 7: 5805. https://doi.org/10.3390/su15075805