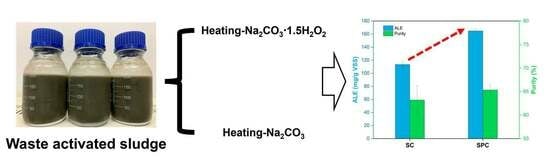
Enhanced Recovery of Alginate-like Extracellular Polymers (ALE) from Waste-Activated Sludge Using Sodium Percarbonate: Performance and Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Waste-Activated Sludge and Reagents
2.2. Extraction Procedures
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Amount and Quality of ALE
3.2. The Variation of Sludge Flocs
3.2.1. The Particle Size Distribution of Sludge Flocs
3.2.2. Composition of Sludge EPS
3.3. The Properties of ALE Products
3.3.1. Monosaccharides Composition of ALE
3.3.2. Surface Functional Groups
3.3.3. UV–Vis Spectrum of ALE
3.3.4. 1H NMR Spectra of ALEs
3.4. Environmental Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhai, Y.; Xu, Z.; Liu, L.; Ren, W.; Xie, Y.; Li, C.; Zhu, Y.; Xu, M. Unraveling the Impacts of Cu+-Based Treatments on Sludge Dewaterability: The Overlooked Role of Cu3+. Chem. Eng. J. 2023, 457, 141106. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Rameez, H.; Farooqi, I.H.; Basheer, F. Recent Advancement in Commercial and Other Sustainable Techniques for Energy and Material Recovery from Sewage Sludge. Water 2023, 15, 948. [Google Scholar] [CrossRef]
- Yu, H.-Q. Molecular Insights into Extracellular Polymeric Substances in Activated Sludge. Environ. Sci. Technol. 2020, 54, 7742–7750. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced Technology Based for Sewage Sludge Deep Dewatering: A Critical Review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, M.; Li, D.; Yang, P.; Xu, S.; Wang, D. Effects of Alkalinity on Interaction between EPS and Hydroxy-Aluminum with Different Speciation in Wastewater Sludge Conditioning with Aluminum Based Inorganic Polymer Flocculant. J. Environ. Sci. 2021, 100, 257–268. [Google Scholar] [CrossRef]
- Bahgat, N.T.; Wilfert, P.; Korving, L.; van Loosdrecht, M. Integrated Resource Recovery from Aerobic Granular Sludge Plants. Water Res. 2023, 234, 119819. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame Retardant Property of Flax Fabrics Coated by Extracellular Polymeric Substances Recovered from Both Activated Sludge and Aerobic Granular Sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lee, Y.-J.; Yuan, T.; Lei, Z.; Adachi, Y.; Zhang, Z.; Lin, Y.; van Loosdrecht, M.C.M. A Review on Recovery of Extracellular Biopolymers from Flocculent and Granular Activated Sludges: Cognition, Key Influencing Factors, Applications, and Challenges. Bioresour. Technol. 2022, 363, 127854. [Google Scholar] [CrossRef]
- Sheng, G.-P.; Yu, H.-Q.; Li, X.-Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Lin, Y.M.; Sharma, P.K.; van Loosdrecht, M.C.M. The Chemical and Mechanical Differences between Alginate-like Exopolysaccharides Isolated from Aerobic Flocculent Sludge and Aerobic Granular Sludge. Water Res. 2013, 47, 57–65. [Google Scholar] [CrossRef]
- Lin, Y.; de Kreuk, M.; van Loosdrecht, M.C.M.; Adin, A. Characterization of Alginate-like Exopolysaccharides Isolated from Aerobic Granular Sludge in Pilot-Plant. Water Res. 2010, 44, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Nierop, K.G.J.; Girbal-Neuhauser, E.; Adriaanse, M.; van Loosdrecht, M.C.M. Sustainable Polysaccharide-Based Biomaterial Recovered from Waste Aerobic Granular Sludge as a Surface Coating Material. Sustain. Mater. Technol. 2015, 4, 24–29. [Google Scholar] [CrossRef]
- de Amorim de Carvalho, C.; Ferreira dos Santos, A.; Tavares Ferreira, T.J.; Sousa Aguiar Lira, V.N.; Mendes Barros, A.R.; Bezerra dos Santos, A. Resource Recovery in Aerobic Granular Sludge Systems: Is It Feasible or Still a Long Way to Go? Chemosphere 2021, 274, 129881. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y. Enhancing Extraction of Alginate like Extracellular Polymers (ALE) from Flocculent Sludge by Surfactants. Sci. Total Environ. 2022, 837, 155673. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.M.; Wang, L.; Chi, Z.M.; Liu, X.Y. Bacterial Alginate Role in Aerobic Granular Bio-particles Formation and Settleability Improvement. Sep. Sci. Technol. 2008, 43, 1642–1652. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Lin, Y.; Malpei, F. Extracellular Polymeric Substances Extraction and Recovery from Anammox Granules: Evaluation of Methods and Protocol Development. Chem. Eng. J. 2019, 374, 112–122. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Wang, D.; Yang, G.; Pan, L.; Wang, Q.; Ni, B.-J.; Li, H.; Yuan, X.; Jiang, L.; et al. Fe(II) Catalyzing Sodium Percarbonate Facilitates the Dewaterability of Waste Activated Sludge: Performance, Mechanism, and Implication. Water Res. 2020, 174, 115626. [Google Scholar] [CrossRef]
- Mo, Z.; Tan, Z.; Liang, J.; Guan, Z.; Liao, X.; Jian, J.; Liu, H.; Li, Y.; Dai, W.; Sun, S. Iron-Rich Sludge Biochar Triggers Sodium Percarbonate Activation for Robust Sulfamethoxazole Removal: Collaborative Roles of Reactive Oxygen Species and Electron Transfer. Chem. Eng. J. 2023, 457, 141150. [Google Scholar] [CrossRef]
- Liang, J.; Tan, Z.; Zhang, L.; Li, C.; Mo, Z.; Ye, M.; Ai, J.; Huang, S.; Sun, S.; Liu, H. Chalcopyrite-Activated Sodium Percarbonate Oxidation for Sludge Dewaterability Enhancement: Synergetic Roles of •OH and 1O2. Chem. Eng. J. 2023, 465, 142863. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Zheng, K.; Guo, H.; Tian, L.; Zhu, T.; Liu, Y. Ultrasound-Sodium Percarbonate Effectively Promotes Short-Chain Carboxylic Acids Production from Sewage Sludge through Anaerobic Fermentation. Bioresour. Technol. 2022, 364, 128024. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, P.; Guo, H.; Zheng, K.; Zhu, T.; Liu, Y. Performance and Mechanism of Sodium Percarbonate (SPC) Enhancing Short-Chain Fatty Acids Production from Anaerobic Waste Activated Sludge Fermentation. J. Environ. Manag. 2022, 313, 115025. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, X.; Wang, F.; Fang, S.; Zhang, L.; Huang, W.; Fang, F.; Cao, J.; Luo, J. Unveiling the Behaviors and Mechanisms of Percarbonate on the Sludge Anaerobic Fermentation for Volatile Fatty Acids Production. Sci. Total Environ. 2022, 838, 156054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, K.; Ding, J.; Guo, H.; Chen, X.; Zhu, T.; Sun, P.; Liu, Y. Ultrasonic Radiation Enhances Percarbonate Oxidation for Improving Anaerobic Digestion of Waste Activated Sludge. Chem. Eng. J. 2023, 457, 141178. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, P.; Guo, H.; Wang, D.; Zhu, T.; Liu, Y. Enhancing Methane Production from Anaerobic Digestion of Waste Activated Sludge through a Novel Sodium Percarbonate (SPC) Pretreatment: Reaction Kinetics and Mechanisms. ACS ES&T Eng. 2022, 2, 1326–1340. [Google Scholar] [CrossRef]
- Ling, X.; Deng, J.; Ye, C.; Cai, A.; Ruan, S.; Chen, M.; Li, X. Fe(II)-Activated Sodium Percarbonate for Improving Sludge Dewaterability: Experimental and Theoretical Investigation Combined with the Evaluation of Subsequent Utilization. Sci. Total Environ. 2021, 799, 149382. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Yang, G.; Yuan, X.; Yuan, L.; Li, Z.; Xu, Q.; Liu, X.; Yang, Q.; Tang, W.; et al. In-Depth Research on Percarbonate Expediting Zero-Valent Iron Corrosion for Conditioning Anaerobically Digested Sludge. J. Hazard. Mater. 2021, 419, 126389. [Google Scholar] [CrossRef]
- Yue, L.; Cheng, J.; Hua, J.; Dong, H.; Zhou, J. A Sodium Percarbonate/Ultraviolet System Generated Free Radicals for Degrading Capsaicin to Alleviate Inhibition of Methane Production during Anaerobic Digestion of Lipids and Food Waste. Sci. Total Environ. 2021, 761, 143269. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y. Controlling Factors and Involved Mechanisms on Forming Alginate like Extracellular Polymers in Flocculent Sludge. Chem. Eng. J. 2022, 439, 135792. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y. Recovery of Extracellular Biopolymers from Conventional Activated Sludge: Potential, Characteristics and Limitation. Water Res. 2021, 205, 117706. [Google Scholar] [CrossRef]
- APHA. Standard Methods For the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2009. [Google Scholar]
- Liu, X.; Zhai, Y.; Xu, Z.; Zhu, Y.; Zhou, Y.; Wang, Z.; Liu, L.; Liang, F.; Ren, W.; Xie, Y.; et al. One-Pot Production of 5-Methylfurfural (5-MF) and Enhanced Dewaterability of Waste Activated Sludge by Hydrothermal Treatment with Natural Deep Eutectic Solvents (NADES): Experimental and Theoretical Studies. Chem. Eng. J. 2023, 464, 142575. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Zhao, Y.; Chai, X.; Niu, D. Enhanced Dewaterability of Sewage Sludge in the Presence of Fe(II)-Activated Persulfate Oxidation. Bioresour. Technol. 2012, 116, 259–265. [Google Scholar] [CrossRef]
- Guo, L.; Lu, M.; Li, Q.; Zhang, J.; Zong, Y.; She, Z. Three-Dimensional Fluorescence Excitation–Emission Matrix (EEM) Spectroscopy with Regional Integration Analysis for Assessing Waste Sludge Hydrolysis Treated with Multi-Enzyme and Thermophilic Bacteria. Bioresour. Technol. 2014, 171, 22–28. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption Spectral Slopes and Slope Ratios as Indicators of Molecular Weight, Source, and Photobleaching of Chromophoric Dissolved Organic Matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef]
- Xing, Y.; Huang, X.; Wang, H.; Yu, J. Insight in the Mechanism of Alkali Treatment Methods Effecting Dewatered Sludge Fermentation from Microbial Characteristics. J. Environ. Chem. Eng. 2022, 10, 108861. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Yu, X.; Yu, P.; Guo, X.; Zhang, B.; Xiao, B. New Insights into the Effect of Thermal Treatment on Sludge Dewaterability. Sci. Total Environ. 2019, 656, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; van Lier, J.B.; de Kreuk, M.K. Effects of Mild Thermal Pre-Treatment Combined with H2O2 Addition on Waste Activated Sludge Digestibility. Waste Manag. 2022, 141, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Aftab, B.; Truong, H.B.; Cho, J.; Hur, J. Enhanced Performance of a Direct Contact Membrane Distillation System via In-Situ Thermally Activated H2O2 Oxidation for the Treatment of Landfill Leachate. J. Membr. Sci. 2022, 652, 120478. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Deng, Y.; Sato, Y.; Wu, D.; Chen, G. Coupling Methane and Bioactive Polysaccharide Recovery from Wasted Activated Sludge: A Sustainable Strategy for Sludge Treatment. Water Res. 2023, 233, 119775. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-B.; Liu, X.-T.; Chen, J.-M.; Peng, D.-C.; He, F. Composition and Functional Group Characterization of Extracellular Polymeric Substances (EPS) in Activated Sludge: The Impacts of Polymerization Degree of Proteinaceous Substrates. Water Res. 2018, 129, 133–142. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Y.; Qian, Z.; Chen, H.; Li, W.; Li, Q.; Xue, S. The Effect of Extracellular Polymeric Substances (EPS) of Iron-Oxidizing Bacteria (Ochrobactrum EEELCW01) on Mineral Transformation and Arsenic (As) Fate. J. Environ. Sci. 2023, 130, 187–196. [Google Scholar] [CrossRef]
- Sha, L.; Wu, Z.; Ling, Z.; Liu, X.; Yu, X.; Zhang, S. Investigation on the Improvement of Activated Sludge Dewaterability Using Different Iron Forms (ZVI vs. Fe(II))/Peroxydisulfate Combined Vertical Electro-Dewatering Processes. Chemosphere 2022, 292, 133416. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Meng, P.; Chen, G.; Guan, Y.; Liu, G. Correlation of Structural Extracellular Polymeric Substances in the Mesh Biofilms with Solids Retention Time and Biofilm Hydraulic Resistance in Dynamic Membrane Bioreactors. Sci. Total Environ. 2022, 832, 155000. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gu, J.; Wei, G.; Ba, J.; Zhang, L.; Li, Z.; Pei, R.; Li, J.; Wei, J. Three-Dimensional Electro-Fenton Degradation of Ciprofloxacin Catalyzed by CuO Doped Red Mud Particle Electrodes: Influencing Factors, Possible Degradation Pathways and Energy Consumption. J. Environ. Chem. Eng. 2022, 10, 107737. [Google Scholar] [CrossRef]
- Yin, C.; Meng, F.; Meng, Y.; Chen, G.-H. Differential Ultraviolet–Visible Absorbance Spectra for Characterizing Metal Ions Binding onto Extracellular Polymeric Substances in Different Mixed Microbial Cultures. Chemosphere 2016, 159, 267–274. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Q.; Du, Z.; Zhang, W.; Wang, D.; Peng, Y. Mechanistic Insights into the Effects of Biopolymer Conversion on Macroscopic Physical Properties of Waste Activated Sludge during Hydrothermal Treatment: Importance of the Maillard Reaction. Sci. Total Environ. 2021, 769, 144798. [Google Scholar] [CrossRef]
- Bartoszek, M.; Polak, J.; Sułkowski, W.W. NMR Study of the Humification Process during Sewage Sludge Treatment. Chemosphere 2008, 73, 1465–1470. [Google Scholar] [CrossRef]
- Pronk, M.; Neu, T.R.; van Loosdrecht, M.C.M.; Lin, Y.M. The Acid Soluble Extracellular Polymeric Substance of Aerobic Granular Sludge Dominated by Defluviicoccus sp. Water Res. 2017, 122, 148–158. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Water content | 98.16 ± 0.14% |
| Total suspended solid (TSS) | 18.04 ± 0.36 g/L |
| Volatile suspended solid (VSS) | 8.93 ± 0.10 g/L |
| pH | 7.04 ± 0.03 |
| Capillary suction time (CST) | 37.9 ± 1.0 s |
| Medium diameter | 33.22 ± 3.26 μm |
| EPS Fractions | Samples | I | II | III | IV | V |
|---|---|---|---|---|---|---|
| S-EPS | RS | 19,076.33 | 14,560.01 | 10,746.11 | 48,497.00 | 17,251.53 |
| SC | 3834.31 | 5955.42 | 8636.46 | 28,679.75 | 29,275.10 | |
| SPC | 2307.45 | 3159.86 | 7290.10 | 14,318.38 | 26,475.90 | |
| LB-EPS | RS | 10,988.54 | 8012.61 | 6263.63 | 14,057.92 | 5123.95 |
| SC | 7730.52 | 9694.10 | 10,094.36 | 21,187.43 | 14,179.75 | |
| SPC | 7020.12 | 7378.08 | 10,213.35 | 14,069.63 | 13,261.43 | |
| TB-EPS | RS | 6836.05 | 5028.91 | 5298.01 | 8150.83 | 4064.37 |
| SC | 7817.05 | 9008.21 | 9380.95 | 17,656.38 | 12,310.77 | |
| SPC | 6405.86 | 5434.81 | 7984.11 | 8160.08 | 8061.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ren, W.; Zhai, Y.; Xie, Y.; Liang, F.; Xu, Z. Enhanced Recovery of Alginate-like Extracellular Polymers (ALE) from Waste-Activated Sludge Using Sodium Percarbonate: Performance and Characteristics. Sustainability 2023, 15, 14573. https://doi.org/10.3390/su151914573
Liu X, Ren W, Zhai Y, Xie Y, Liang F, Xu Z. Enhanced Recovery of Alginate-like Extracellular Polymers (ALE) from Waste-Activated Sludge Using Sodium Percarbonate: Performance and Characteristics. Sustainability. 2023; 15(19):14573. https://doi.org/10.3390/su151914573
Chicago/Turabian StyleLiu, Xiaoping, Wanying Ren, Yunbo Zhai, Yu Xie, Fashen Liang, and Zhixiang Xu. 2023. "Enhanced Recovery of Alginate-like Extracellular Polymers (ALE) from Waste-Activated Sludge Using Sodium Percarbonate: Performance and Characteristics" Sustainability 15, no. 19: 14573. https://doi.org/10.3390/su151914573