Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol
Abstract
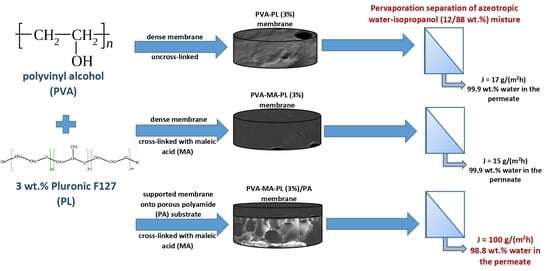
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.2.1. Dense Membranes
2.2.2. Porous Membranes
2.2.3. Supported Membranes
2.3. Membrane Investigation Methods
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Atomic Force Microscopy (AFM)
2.3.3. FTIR Spectroscopy (FTIR)
2.3.4. Ultrafiltration
2.3.5. Pervaporation
2.3.6. Contact Angle Determination
2.3.7. Swelling Experiment
3. Results and Discussion
3.1. Development and Study of Dense PVA Membranes
3.1.1. Transport Properties of Dense PVA Membranes Modified by Pluronic F127
3.1.2. Structure Characteristics of Dense PVA Membranes Modified by Pluronic F127
3.2. Development and Study of Porous PA Membranes (Substrates)
3.2.1. Structure of PA membranes (substrates)
3.2.2. Transport Properties of PA Membranes (Substrates)
3.3. Development and Study of Supported PVA Membranes
3.3.1. Transport Properties of Supported PVA/PA Membranes
3.3.2. Transport Properties of Supported PVA-MA-PL (3 wt.%)/PA-17 Membrane
3.3.3. Study the Stability of the Supported PVA-MA-PL (3 wt.%)/PA-17 Membrane
3.4. Comparison of Supported PVA-MA-PL (3 wt.%)/PA-17 Membrane Performance with Other Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzminova, A.; Dmitrenko, M.; Mazur, A.; Ermakov, S.; Penkova, A. Novel Pervaporation Membranes Based on Biopolymer Sodium Alginate Modified by FeBTC for Isopropanol Dehydration. Sustainability 2021, 13, 6092. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Esmailinezhad, M.; Drioli, E. Effect of polymer composition in PEEKWC/PVP blends on pervaporation separation of ethanol/cyclohexane mixture. Sep. Purif. Technol. 2010, 75, 257–265. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Mazur, A.; Semenov, K.; Solovyev, N.; Penkova, A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers 2022, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, H.; Peng, F.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Memb. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Smitha, B. Separation of organic–organic mixtures by pervaporation? A review. J. Memb. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Ortiz, I.; Urtiaga, A.; Ibáñez, R.; Gómez, P.; Gorri, D. Laboratory and pilot plant-scale study on the dehydration of cyclohexane by pervaporation. J. Chem. Technol. Biotechnol. 2006, 81, 48–57. [Google Scholar] [CrossRef]
- Li, J. Laboratory and pilot-scale study on dehydration of benzene by pervaporation. J. Memb. Sci. 2002, 203, 127–136. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Memb. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Hu, C.; Li, B.; Guo, R.; Wu, H.; Jiang, Z. Pervaporation performance of chitosan–poly(acrylic acid) polyelectrolyte complex membranes for dehydration of ethylene glycol aqueous solution. Sep. Purif. Technol. 2007, 55, 327–334. [Google Scholar] [CrossRef]
- Peng, M.; Vane, L.M.; Liu, S.X. Recent advances in VOCs removal from water by pervaporation. J. Hazard. Mater. 2003, 98, 69–90. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Pan, F.; Peng, F.; Wu, H. The unusual change of permeation rate in PDMS membranes filled with crystalline calixarene and its derivative. J. Memb. Sci. 2006, 279, 111–119. [Google Scholar] [CrossRef]
- Wilson, I.D. Encyclopedia of Separation Science, 1st ed.; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2000; ISBN 978-0-12-226770-3. [Google Scholar]
- Lide David, R. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, 79th ed.; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-0468-2. [Google Scholar]
- Othmer, K. Encyclopedia of Chemical Technology, Volumes 1-26; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 0471484962. [Google Scholar]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Adoor, S.G.; Sairam, M.; Manjeshwar, L.S.; Raju, K.V.S.N.; Aminabhavi, T.M. Sodium montmorillonite clay loaded novel mixed matrix membranes of poly (vinyl alcohol) for pervaporation dehydration of aqueous mixtures of isopropanol and 1,4-dioxane. J. Membr. Sci. 2006, 285, 182–195. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairam, M.; Naidu, B.V.K.; Nataraj, S.K.; Sreedhar, B.; Aminabhavi, T.M. Poly(vinyl alcohol)-iron oxide nanocomposite membranes for pervaporation dehydration of isopropanol, 1,4-dioxane and tetrahydrofuran. J. Memb. Sci. 2006, 283, 65–73. [Google Scholar] [CrossRef]
- Sairam, M.; Patil, M.; Veerapur, R.; Patil, S.; Aminabhavi, T. Novel dense poly(vinyl alcohol)–TiO2 mixed matrix membranes for pervaporation separation of water–isopropanol mixtures at 30 °C. J. Memb. Sci. 2006, 281, 95–102. [Google Scholar] [CrossRef]
- Mosleh, S.; Khosravi, T.; Bakhtiari, O.; Mohammadi, T. Zeolite filled polyimide membranes for dehydration of isopropanol through pervaporation process. Chem. Eng. Res. Des. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.P.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2019, 35, 565–590. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zhou, S.; Xue, A.; Zhang, Y.; Zhao, Y.; Zhong, J.; Zhang, Q. Graphitic carbon nitride nanosheets embedded in poly(vinyl alcohol) nanocomposite membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2017, 188, 24–37. [Google Scholar] [CrossRef]
- Ghobadi, N.; Mohammadi, T.; Kasiri, N.; Kazemimoghadam, M. Modified poly(vinyl alcohol)/chitosan blended membranes for isopropanol dehydration via pervaporation: Synthesis optimization and modeling by response surface methodology. J. Appl. Polym. Sci. 2017, 134, 44587. [Google Scholar] [CrossRef]
- Yeom, C.K.; Jegal, J.G.; Lee, K.H. Characterization of relaxation phenomena and permeation behaviors in sodium alginate membrane during pervaporation separation of ethanol-water mixture. J. Appl. Polym. Sci. 1996, 62, 1561–1576. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- Razmgar, K.; Nasiraee, M. Polyvinyl alcohol-based membranes for filtration of aqueous solutions: A comprehensive review. Polym. Eng. Sci. 2022, 62, 25–43. [Google Scholar] [CrossRef]
- Shiue, A.; Yin, M.-J.; Tsai, M.-H.; Chang, S.-M.; Leggett, G. Carbon Dioxide Separation by Polyethylene Glycol and Glutamic Acid/Polyvinyl Alcohol Composite Membrane. Sustainability 2021, 13, 13367. [Google Scholar] [CrossRef]
- Kurşun, F.; Işıklan, N. Development of thermo-responsive poly(vinyl alcohol)-g-poly(N-isopropylacrylamide) copolymeric membranes for separation of isopropyl alcohol/water mixtures via pervaporation. J. Ind. Eng. Chem. 2016, 41, 91–104. [Google Scholar] [CrossRef]
- Dave, H.K.; Nath, K. Synthesis, Characterization and Application of Disodium Tetraborate Cross-Linked Polyvinyl Alcohol Membranes for Pervaporation Dehydration of Ethylene Glycol. Acta Chim. Slov. 2018, 65, 902–918. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Missyul, A.B.; Kuzminova, A.I.; Markelov, D.A.; Ermakov, S.S.; Roizard, D. Development and investigation of mixed-matrix PVA-fullerenol membranes for acetic acid dehydration by pervaporation. Sep. Purif. Technol. 2017, 187, 285–293. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Development of novel grafted hybrid PVA membranes using glycidyltrimethylammonium chloride for pervaporation separation of water–isopropanol mixtures. J. Ind. Eng. Chem. 2013, 19, 427–437. [Google Scholar] [CrossRef]
- Svang-Ariyaskul, A.; Huang, R.Y.M.; Douglas, P.L.; Pal, R.; Feng, X.; Chen, P.; Liu, L. Blended chitosan and polyvinyl alcohol membranes for the pervaporation dehydration of isopropanol. J. Memb. Sci. 2006, 280, 815–823. [Google Scholar] [CrossRef]
- Suhas, D.P.; Aminabhavi, T.M.; Raghu, A.V. Mixed matrix membranes of H-ZSM5-loaded poly(vinyl alcohol) used in pervaporation dehydration of alcohols: Influence of silica/alumina ratio. Polym. Eng. Sci. 2014, 54, 1774–1782. [Google Scholar] [CrossRef]
- Naidu, B.V.K.; Sairam, M.; Raju, K.V.S.N.; Aminabhavi, T.M. Pervaporation separation of water + isopropanol mixtures using novel nanocomposite membranes of poly(vinyl alcohol) and polyaniline. J. Memb. Sci. 2005. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Memb. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Galiano, F.; Drioli, E. Pervaporation separation of ethanol/ETBE mixture using poly(lactic acid)/poly(vinyl pyrrolidone) blend membranes. J. Memb. Sci. 2011, 373, 29–35. [Google Scholar] [CrossRef]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.; Singh, R.P.; Choudhary, V. Selective polydimethylsiloxane/polyimide blended IPN pervaporation membrane for methanol/toluene azeotrope separation. Sep. Purif. Technol. 2011, 76, 407–418. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwon, Y.; Moon, M.; Shon, M.; Nam, S.; Park, Y. Poly(vinyl alcohol) and poly(vinyl amine) blend membranes for isopropanol dehydration. J. Appl. Polym. Sci. 2017, 134, 45572. [Google Scholar] [CrossRef]
- Golemme, G.; Bruno, A.; Manes, R.; Muoio, D. Preparation and properties of superglassy polymers—zeolite mixed matrix membranes. Desalination 2006, 200, 440–442. [Google Scholar] [CrossRef]
- Teli, S.B.; Gokavi, G.S.; Sairam, M.; Aminabhavi, T.M. Mixed matrix membranes of poly(vinyl alcohol) loaded with phosphomolybdic heteropolyacid for the pervaporation separation of water–isopropanol mixtures. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 55–62. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Carn, F.; Tavares, S.R.; Menguy, N.; Giménez-Marqués, M.; Bellido, E.; Horcajada, P.; Berthelot, T.; Kuzminova, A.I.; et al. Covalent and Selective Grafting of Polyethylene Glycol Brushes at the Surface of ZIF-8 for the Processing of Membranes for Pervaporation. ACS Sustain. Chem. Eng. 2019, 7, 6629–6639. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Sokolova, M.P.; Chen, B.; Plisko, T.V.; Markelov, D.A.; Ermakov, S.S. Impact of fullerene loading on the structure and transport properties of polysulfone mixed-matrix membranes. J. Mater. Sci. 2016, 51, 7652–7659. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Atta, R.R.; Zolotarev, A.A.; Mazur, A.S.; Vezo, O.S.; Lahderanta, E.; Markelov, D.A.; Ermakov, S.S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Kim, S.; Pechar, T.W.; Marand, E. Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 192, 330–339. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Burts, K.S.; Plisko, T.V.; Bildyukevich, A.V.; Penkova, A.V.; Pratsenko, S.A. Modification of polysulfone ultrafiltration membranes using block copolymer Pluronic F127. Polym. Bull. 2021, 78, 6549–6576. [Google Scholar] [CrossRef]
- Polak, D.; Sułkowska, J.; Szwast, M. The influence of surfactant Pluronic P123 addition on the mixed matrix membrane PEBAX 2533—ZIF-8 separation properties. Desalin. WATER Treat. 2021, 214, 64–73. [Google Scholar] [CrossRef]
- Plisko, T.V.; Penkova, A.V.; Burts, K.S.; Bildyukevich, A.V.; Dmitrenko, M.E.; Melnikova, G.B.; Atta, R.R.; Mazur, A.S.; Zolotarev, A.A.; Missyul, A.B. Effect of Pluronic F127 on porous and dense membrane structure formation via non-solvent induced and evaporation induced phase separation. J. Memb. Sci. 2019, 580, 336–349. [Google Scholar] [CrossRef]
- Mulijani, S.; Mulanawati, A. Enhanced Performance of Asymmetric Polystyrene Membrane by Incorporation of Pluronic F127 and Its Application for Pervaporation Separation. Procedia Chem. 2012, 4, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Semsarzadeh, M.A.; Ghalei, B. Characterization and gas permeability of polyurethane and polyvinyl acetate blend membranes with polyethylene oxide–polypropylene oxide block copolymer. J. Memb. Sci. 2012, 401–402, 97–108. [Google Scholar] [CrossRef]
- Li, B.; Zhao, W.; Su, Y.; Jiang, Z.; Dong, X.; Liu, W. Enhanced desulfurization performance and swelling resistance of asymmetric hydrophilic pervaporation membrane prepared through surface segregation technique. J. Memb. Sci. 2009, 326, 556–563. [Google Scholar] [CrossRef]
- Dmitrenko, M.E.; Penkova, A.V.; Atta, R.R.; Zolotarev, A.A.; Plisko, T.V.; Mazur, A.S.; Solovyev, N.D.; Ermakov, S.S. The development and study of novel membrane materials based on polyphenylene isophthalamide—Pluronic F127 composite. Mater. Des. 2019, 165, 107596. [Google Scholar] [CrossRef]
- Schildknecht, C.E. Polyvinyl alcohol, properties and applications, C.A. Finch, Wiley, New York, 1973. 622 pp. $37.50. J. Polym. Sci. Polym. Lett. Ed. 1974, 12, 105–106. [Google Scholar] [CrossRef]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the Crosslinking of Poly (Vinyl Alcohol). J. Polym. Res. 2006, 13, 161–169. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Chen, B.; Semenov, K.N.; Kroto, H.W. Transport properties of cross-linked fullerenol–PVA membranes. Carbon N. Y. 2014, 76, 446–450. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; El Damrawi, G.; Al-Shahawy, A. Some studies on calcium phosphate embedded in polyvinyl alcohol matrix before and after γ-irradiation. Phys. B Condens. Matter. 2010, 405, 808–816. [Google Scholar] [CrossRef]
- Omkaram, I.; Sreekanth Chakradhar, R.P.; Lakshmana Rao, J. EPR, optical, infrared and Raman studies of VO2+ ions in polyvinylalcohol films. Phys. B Condens. Matter. 2007, 388, 318–325. [Google Scholar] [CrossRef]
- Pirzada, T.; Arvidson, S.A.; Saquing, C.D.; Shah, S.S.; Khan, S.A. Hybrid silica-PVA nanofibers via sol-gel electrospinning. Langmuir 2012, 28, 5834–5844. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Abdelrazek, E.M.; Tarabiah, A.E. Modeling and Physical Properties of Lead Sulphide/Polyvinyl Alcohol Nano-Composite. Quantum Matter. 2016, 5, 257–262. [Google Scholar] [CrossRef]
- Xie, Z.; Hoang, M.; Duong, T.; Ng, D.; Dao, B.; Gray, S. Sol–gel derived poly(vinyl alcohol)/maleic acid/silica hybrid membrane for desalination by pervaporation. J. Memb. Sci. 2011, 383, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Young, T.-H.; Chen, L.-W. Pore formation mechanism of membranes from phase inversion process. Desalination 1995, 103, 233–247. [Google Scholar] [CrossRef]
- McKelvey, S. Phase separation, vitrification, and the manifestation of macrovoids in polymeric asymmetric membranes. J. Memb. Sci. 1996, 112, 29–39. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Sha, F.; Yang, H.; Zhang, Q.; Shi, S.; Zheng, Z. Investigation of the Porosity Distribution, Permeability, and Mechanical Performance of Pervious Concretes. Processes 2018, 6, 78. [Google Scholar] [CrossRef] [Green Version]
- Elahi, S.H.; Escobar, I.C. Investigation of the Effects of Thickness and Presence of Pore Formers on Tailor-Made Ultrafiltration Polysulfone Membranes. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; pp. 271–283. ISBN 9780841226180. [Google Scholar]
- Wang, J.; Zhang, W.; Li, W.; Xing, W. Preparation and characterization of chitosan-poly (vinyl alcohol)/polyvinylidene fluoride hollow fiber composite membranes for pervaporation dehydration of isopropanol. Korean J. Chem. Eng. 2015, 32, 1369–1376. [Google Scholar] [CrossRef]
- Mali, M.G.; Magalad, V.T.; Gokavi, G.S.; Aminabhavi, T.M.; Raju, K.V.S.N. Pervaporation separation of isopropanol-water mixtures using mixed matrix blend membranes of poly(vinyl alcohol)/poly(vinyl pyrrolidone) loaded with phosphomolybdic acid. J. Appl. Polym. Sci. 2011, 121, 711–719. [Google Scholar] [CrossRef]
- Burshe, M.C.; Sawant, S.B.; Joshi, J.B.; Pangarkar, V.G. Separation Sorption and permeation of binary water-alcohol systems through PVA membranes crosslinked with multifunctional crosslinking agents. Sep. Purif. Technol. 1997, 12, 145–156. [Google Scholar] [CrossRef]
- Rao, K.S.V.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabhavi, T.M. Blend membranes of chitosan and poly(vinyl alcohol) in pervaporation dehydration of isopropanol and tetrahydrofuran. J. Appl. Polym. Sci. 2007, 103, 1918–1926. [Google Scholar] [CrossRef]
- Kurkuri, M.D.; Toti, U.S.; Aminabhavi, T.M. Syntheses and characterization of blend membranes of sodium alginate and poly(vinyl alcohol) for the pervaporation separation of water + isopropanol mixtures. J. Appl. Polym. Sci. 2002, 86, 3642–3651. [Google Scholar] [CrossRef]















| Membrane | Type | Cross-Linker | Modifier | Casting Solution Concentration (wt.%) | ||
|---|---|---|---|---|---|---|
| Type | Content (wt.%) | Type | Content (wt.%) | |||
| PVA | Dense | - | - | - | - | 2 |
| PVA-PL (1 wt.%) | Dense | - | - | PL F127 | 1 | 2 |
| PVA-PL (2 wt.%) | Dense | - | - | PL F127 | 2 | 2 |
| PVA-PL (3 wt.%) | Dense | - | - | PL F127 | 3 | 2 |
| PVA-MA | Dense | MA | 35 | - | - | 2 |
| PVA-MA-PL (1 wt.%) | Dense | MA | 35 | PL F127 | 1 | 2 |
| PVA-MA-PL (2 wt.%) | Dense | MA | 35 | PL F127 | 2 | 2 |
| PVA-MA-PL (3 wt.%) | Dense | MA | 35 | PL F127 | 3 | 2 |
| PA-12 | Porous | - | - | - | - | 12 |
| PA-15 | Porous | - | - | - | - | 15 |
| PA-17 | Porous | - | - | - | - | 17 |
| PA-20 | Porous | - | - | - | - | 20 |
| PVA/PA-12 | Supported | - | - | - | - | 2/12 |
| PVA/PA-15 | Supported | - | - | - | - | 2/15 |
| PVA/PA-17 | Supported | - | - | - | - | 2/17 |
| PVA/PA-20 | Supported | - | - | - | - | 2/20 |
| PVA-MA/PA-17 | Supported | MA | 35 | - | - | 2/17 |
| PVA-MA-PL (3 wt.%)/PA-17 | Supported | MA | 35 | PL F127 | 3 | 2/17 |
| Membrane | Surface Parameters | |
|---|---|---|
| Average Roughness, Ra (nm) | Root-Mean-Squared Roughness, Rq (nm) | |
| PA-12 | 30.3 | 37.9 |
| PA-15 | 24.0 | 30.2 |
| PA-17 | 18.7 | 24.0 |
| PA-20 | 4.20 | 5.30 |
| Membrane (Substrate) | Pure Water Flux (JW), l/(m2h) | Flux of BSA (J), l/(m2 h) | R, % | FRR, % |
|---|---|---|---|---|
| PA-12 | 580 | 276 | 33 | 58 |
| PA-15 | 392 | 123 | 82 | 70 |
| PA-17 | 233 | 76 | 99 | 76 |
| PA-20 | 63 | 59 | 99 | 87 |
| Membrane | Cross- Linker | Modifier | Support | Water in Feed, wt.% | T, °C | Permeation Flux, kg/(m2h) | Separation Factor (β) | Reference |
|---|---|---|---|---|---|---|---|---|
| PVA-MA-PL (3 wt.%)/PA-17 | MA | PL F127 | PA | 12 | 22 | 0.100 | 589 | This work |
| CS-PVA/PVDF | GA | CS | PVDF | 10 | 30 | 0.070 | 683 | [67] |
| PVA/PVP-PMA | GA | PMA | - | 10 | 30 | 0.036 | 29,991 | [68] |
| PVA-CA | CA | - | - | 10 | 30 | 0.053 | 291 | [69] |
| PVA-USF | USF | - | - | 10 | 30 | 0.095 | 77 | [70] |
| PVA/GA | GA | - | - | 10 | 30 | 0.040 | 21 | [71] |
| PVA-NaAlg (75:25)/GA | GA | - | - | 10 | 30 | 0.039 | 91 | |
| PVA-MA-PL (3 wt.%)/PA-17 | MA | PL F127 | PA | 20 | 22 | 0.296 | 218 | This work |
| PVA-CS | MA | CS | UPM-20 | 20 | 22 | 0.233 | 69 | [17] |
| PVA-fullerenol (5 wt.%)-CS (20 wt.%) | MA | CS/Fullerenol | UPM-20 | 20 | 22 | 0.241 | 121 | |
| PVA-fullerenol (5 wt.%)-CS (20 wt.%)/LbL-5PSS, CS | MA | CS/Fullerenol | UPM-20 | 20 | 22 | 0.340 | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Atta, R.; Zolotarev, A.; Kuzminova, A.; Ermakov, S.; Penkova, A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability 2022, 14, 3561. https://doi.org/10.3390/su14063561
Dmitrenko M, Atta R, Zolotarev A, Kuzminova A, Ermakov S, Penkova A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability. 2022; 14(6):3561. https://doi.org/10.3390/su14063561
Chicago/Turabian StyleDmitrenko, Mariia, Ramadan Atta, Andrey Zolotarev, Anna Kuzminova, Sergey Ermakov, and Anastasia Penkova. 2022. "Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol" Sustainability 14, no. 6: 3561. https://doi.org/10.3390/su14063561