Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies
Abstract
:1. Introduction
2. Materials and Methods
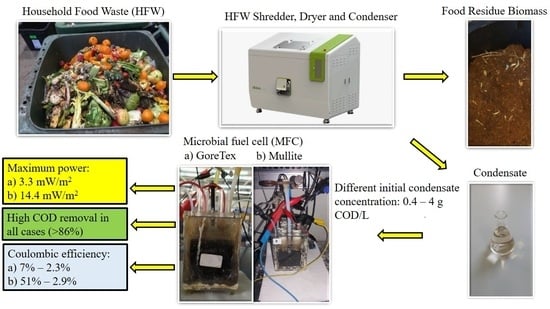
2.1. MFCs Set Up and Operation
2.2. Analytical Methods, Calculations and Electrochemical Characterization
3. Results and Discussion
3.1. MFC Operation at Different Initial Condensate Concentrations
3.2. Linear Sweep Voltammetry Experiments at Different Initial Condensate Concentrations
3.3. E. Lectrochemical Impedance Spectroscopy Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bio-Waste in Europe—Turning Challenges into Opportunities—European Environment Agency. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 2 November 2021).
- Sridhar, A.; Ponnuchamy, M.; Kapoor, A.; Prabhakar, S. Valorization of food waste as adsorbents for toxic dye removal from contaminated waters: A review. J. Hazard. Mater. 2021, 424, 127432. [Google Scholar] [CrossRef] [PubMed]
- Talan, A.; Tiwari, B.; Yadav, B.; Tyagi, R.; Wong, J.; Drogui, P. Food waste valorization: Energy production using novel integrated systems. Bioresour. Technol. 2020, 322, 124538. [Google Scholar] [CrossRef] [PubMed]
- Tremouli, A.; Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G. Exploitation of Digestate from Thermophilic and Mesophilic Anaerobic Digesters Fed with Fermentable Food Waste Using the MFC Technology. Waste Biomass-Valorization 2021, 12, 5361–5370. [Google Scholar] [CrossRef]
- FAO. Food Wastage Footprint & Climate Change. 2015, pp. 1–4. Available online: http://www.fao.org/3/a-bb144e.pdf (accessed on 15 November 2021).
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2019, 299, 122580. [Google Scholar] [CrossRef]
- Venus, J.; Fiore, S.; Demichelis, F.; Pleissner, D. Centralized and decentralized utilization of organic residues for lactic acid production. J. Clean. Prod. 2018, 172, 778–785. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. Bio-augmentation to improve lactic acid production from source-sorted organic household waste. J. Clean. Prod. 2020, 279, 123714. [Google Scholar] [CrossRef]
- Rousta, K.; Bolton, K. Sorting Household Waste at the Source. In Sustainable Resource Recovery and Zero Waste Approaches, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 105–114. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Alexandropoulou, M.; Ntaikou, I.; Lyberatos, G. From waste to fuel: Energy recovery from household food waste via its bioconversion to energy carriers based on microbiological processes. Sci. Total Environ. 2020, 732, 139230. [Google Scholar] [CrossRef]
- WASTE4think-Moving towards Life Cycle Thinking by Integrating Advanced Waste Management Systems. Available online: https://waste4think.eu/ (accessed on 2 November 2021).
- Lytras, G.; Koutroumanou, E.; Lyberatos, G. Anaerobic co-digestion of condensate produced from drying of Household Food Waste and Waste Activated Sludge. J. Environ. Chem. Eng. 2020, 8, 103947. [Google Scholar] [CrossRef]
- Michalopoulos, I.; Lytras, G.; Mathioudakis, D.; Lytras, C.; Goumenos, A.; Zacharopoulos, I.; Papadopoulou, K.; Lyberatos, G. Hydrogen and Methane Production from Food Residue Biomass Product (FORBI). Waste Biomass-Valorization 2019, 11, 1647–1655. [Google Scholar] [CrossRef]
- Skiadas, I.V.; Lyberatos, G. The periodic anaerobic baffled reactor. Water Sci. Technol. 1998, 38, 401–408. [Google Scholar] [CrossRef]
- Tremouli, A.; Karydogiannis, I.; Pandis, P.; Papadopoulou, K.; Argirusis, C.; Stathopoulos, V.N.; Lyberatos, G. Bioelectricity production from fermentable household waste extract using a single chamber microbial fuel cell. Energy Procedia 2019, 161, 2–9. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Genet. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Duan, C.; Duan, W.; Sun, F.; Cui, H.; Zhang, S.; Chen, X. Role of electrode materials on performance and microbial characteristics in the constructed wetland coupled microbial fuel cell (CW-MFC): A review. J. Clean. Prod. 2021, 301, 126951. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ghangrekar, M.M. Preparation of a fouling-resistant sustainable cathode for a single-chambered microbial fuel cell. Water Sci. Technol. 2013, 69, 634–639. [Google Scholar] [CrossRef]
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, V.; Mohebbi-Kalhori, D.; Samimi, A. Ceramic-based microbial fuel cells (MFCs): A review. Int. J. Hydrogen Energy 2017, 42, 1672–1690. [Google Scholar] [CrossRef]
- Tremouli, A.; Greenman, J.; Ieropoulos, I. Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 2018, 123, 19–25. [Google Scholar] [CrossRef]
- Santoro, C.; Flores-Cadengo, C.; Soavi, F.; Kodali, M.; Jimenez, I.M.; Gajda, I.; Greenman, J.; Ieropoulos, I.; Atanassov, P. Ceramic Microbial Fuel Cells Stack: Power generation in standard and supercapacitive mode. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, C.; Geng, S. Membrane-less cloth cathode assembly (CCA) for scalable microbial fuel cells. Biosens. Bioelectron. 2009, 24, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Ntaikou, I.; Menis, N.; Alexandropoulou, M.; Antonopoulou, G.; Lyberatos, G. Valorization of kitchen biowaste for ethanol production via simultaneous saccharification and fermentation using co-cultures of the yeasts Saccharomyces cerevisiae and Pichia stipitis. Bioresour. Technol. 2018, 263, 75–83. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewateri, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; ISBN 978-0875532356. [Google Scholar]
- Tremouli, A.; Pandis, P.K.; Kamperidis, T.; Stathopoulos, V.N.; Argirusis, C.; Lyberatos, G. Performance assessment of a four-air cathode membraneless microbial fuel cell stack for wastewater treatment and energy extraction. E3S Web Conf. 2019, 116, 00093. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Antonopoulou, G.; Tremouli, A.; Lyberatos, G. Production of Gaseous Biofuels and Electricity from Cheese Whey. Ind. Eng. Chem. Res. 2011, 50, 639–644. [Google Scholar] [CrossRef]
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 2010, 79, 228–233. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cheng, S.; Logan, B.E. Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresour. Technol. 2011, 102, 4468–4473. [Google Scholar] [CrossRef]
- Ramasamy, N.S.A.R.P. Electrochemical Impedance Spectroscopy for Microbial Fuel Cell Characterization. J. Microb. Biochem. Technol. 2013, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Savadogo, O.; Guiot, S.R.; Tartakovsky, B. Electrochemical characterization of anodic biofilm development in a microbial fuel cell. J. Appl. Electrochem. 2013, 43, 533–540. [Google Scholar] [CrossRef]
- Wei, B.; Tokash, J.; Zhang, F.; Kim, Y.; Logan, B.E. Electrochemical analysis of separators used in single-chamber, air-cathode microbial fuel cells. Electrochim. Acta 2013, 89, 45–51. [Google Scholar] [CrossRef]
- Uria, N.; Ferrera, I.; Mas, J. Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms. BMC Microbiol. 2017, 17, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, G.; Ghangrekar, M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef]




 , 800
, 800  , 1400
, 1400  and 3000
and 3000  .
.
 , 800
, 800  , 1400
, 1400  and 3000
and 3000  .
.
 , 800
, 800  , 1400
, 1400  and 3000
and 3000  .
.
 , 800
, 800  , 1400
, 1400  and 3000
and 3000  .
.


| Anaerobic Sludge | pH | Conductivity (mS/cm) | Soluble COD (mg/L) | Total COD (mg/L) | TSS (g/L) | VSS (g/L) |
|---|---|---|---|---|---|---|
| 7.3 | 4.4 | 470 | 21,900 | 49 | 21 |
| Cycle Number | mg COD/L | pH | Conductivity (mS/cm) |
|---|---|---|---|
| 1st | 400 | 6.7 | 5.9 |
| 2nd | 500 | 6.9 | 5.0 |
| 3rd | 600 | 6.6 | 5.0 |
| 4th | 800 | 6.5 | 4.9 |
| 5th | 1200 | 6.6 | 4.9 |
| 6th | 1400 | 6.6 | 5.2 |
| 7th | 3000 | 5.1 | 5.4 |
| 8th | 4000 | 4.8 | 3.8 |
| Initial Condensate Concentration (mg COD/L) | Δt Cycle (h) | COD Removal | Elyield (mJ/g COD/L) | |||
|---|---|---|---|---|---|---|
| Cell 1 | Cell 2 | Cell 1 | Cell 2 | Cell 1 | Cell 2 | |
| 400 | 34 | 140 | 90% | 89% | 1.31 | 25.9 |
| 500 | 35 | 96 | 91% | 99% | 1.51 | 12.2 |
| 600 | 36 | 113 | 93% | 93% | 0.53 | 15.3 |
| 800 | 49 | 164 | 95% | 95% | 0.77 | 6.8 |
| 1200 | 57 | 179 | 97% | 96% | 0.51 | 5.8 |
| 1400 | 54 | 164 | 92% | 99% | 0.46 | 7.9 |
| 3000 | 113 | 148 | 86% | 89% | 0.53 | 0.9 |
| 4000 | 121 | 152 | 98% | 87% | 0.50 | 0.5 |
| Fitted Parameters | mg COD/L | |||
|---|---|---|---|---|
| 600 | 800 | 1400 | 3000 | |
| RS (Ω) | 330 | 488 | 551 | 741 |
| RBF (Ω) | 349 | 347 | 354 | 349 |
| RCT (Ω) | 119 | 150 | 173 | 293 |
| Rint (Ω) | 798 | 985 | 1078 | 1383 |
| Fitted Parameters | mg COD/L | |||
|---|---|---|---|---|
| 600 | 800 | 1400 | 3000 | |
| RS (Ω) | 19 | 24 | 27 | 30 |
| RBF (Ω) | 31 | 33 | 35 | 34 |
| RCT (Ω) | 45 | 49 | 52 | 56 |
| Rint (Ω) | 95 | 106 | 114 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamperidis, T.; Pandis, P.K.; Argirusis, C.; Lyberatos, G.; Tremouli, A. Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies. Sustainability 2022, 14, 2625. https://doi.org/10.3390/su14052625
Kamperidis T, Pandis PK, Argirusis C, Lyberatos G, Tremouli A. Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies. Sustainability. 2022; 14(5):2625. https://doi.org/10.3390/su14052625
Chicago/Turabian StyleKamperidis, Theofilos, Pavlos K. Pandis, Christos Argirusis, Gerasimos Lyberatos, and Asimina Tremouli. 2022. "Effect of Food Waste Condensate Concentration on the Performance of Microbial Fuel Cells with Different Cathode Assemblies" Sustainability 14, no. 5: 2625. https://doi.org/10.3390/su14052625