Morphological, Leaf Nutrient, and Fruit Quality Characteristics of Diverse Tomato Cultivars under Organic Low-Input Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Climatic Description
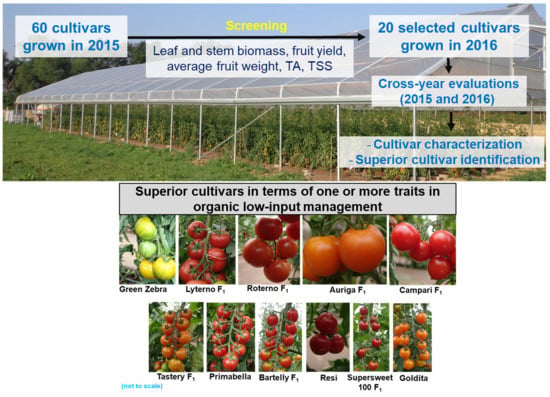
2.2. Cultivars
2.3. Experimental Layout and Crop Cultivation
2.4. Sample Collection, Mineral Nutrient, and Quality Determination
2.4.1. Plant Morphology
2.4.2. Macronutrient Concentrations in Leaves and Fruit
2.4.3. Fruit Dry Matter (DM), Total Soluble Solids (TSS), and Titratable Acidity (TA)
2.4.4. Total Phenolic Concentration (TPC)
2.4.5. Fruit Colour
2.5. Statistical Analyses
3. Results
3.1. Variation of 60 Cultivars
3.2. Morphological and Fruit Quality Characteristics of the 20 Selected Cultivars
3.2.1. Plant Morphological Characteristics
3.2.2. Leaf Macronutrient Concentration
3.2.3. Fruit Minerals
3.2.4. Fruit Quality Characteristics and Fruit Colour
3.2.5. Correlation between Fruit Yield and Quality
4. Discussion
4.1. Genotypic Variability of 60 Tomato Cultivars
4.2. Morphological and Fruit Quality Characterisation of the 20 Selected Cultivars
4.3. Trade-Offs between Yield and Fruit Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Crop Production Quantity by Regions. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 9 February 2020).
- Aldrich, H.T.; Salandanan, K.; Kendall, P.; Bunning, M.; Stonaker, F.; Külen, O.; Stushnoff, C. Cultivar choice provides options for local production of organic and conventionally produced tomatoes with higher quality and antioxidant content. J. Sci. Food Agric. 2010, 90, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.J.S.; Sala, F.C.; Cardoso, J.C. Performance and selection of tomato cultivars for organic cultivation in greenhouse. Rev. Ceres 2019, 66, 94–101. [Google Scholar] [CrossRef]
- Stolz, H.; Stolze, M.; Hamm, U.; Janssen, M.; Ruto, E. Consumer attitudes towards organic versus conventional food with specific quality attributes. NJAS-Wagening. J. Life Sci. 2011, 58, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Lammerts vans Bueren, E.T.; Jones, S.S.; Tamm, L.; Murphy, K.M.; Myers, J.R.; Leifert, C.; Messmer, M.M. The need to breed crop varieties suitable for organic farming, using wheat, tomato and broccoli as examples: A review. NJAS-Wagening. J. Life Sci. 2011, 58, 193–205. [Google Scholar] [CrossRef]
- Horneburg, B.; Myers, J.R. Tomato: Breeding for improved disease resistance in fresh market and home garden varieties. In Organic Crop Breeding; Lammerts van Bueren, E.T., Myers, J.R., Eds.; Wiley-Blackwell: Chichester, UK; Ames, IA, USA, 2012; pp. 239–249. ISBN 9781119945932. [Google Scholar]
- Schouten, H.J.; Tikunov, Y.; Verkerke, W.; Finkers, R.; Bovy, A.; Bai, Y.; Visser, R.G.F. Breeding has increased the diversity of cultivated tomato in the Netherlands. Front. Plant Sci. 2019, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16 (Suppl 1), S181–S189. [Google Scholar] [CrossRef]
- Scott, J.W.; Myers, J.R.; Boches, P.S.; Nichols, C.G.; Angell, F.F. Classical genetics and traditional breeding. In Genetics, Genomics and Breeding of Tomato; Liedl, B.E., Ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 1578088046. [Google Scholar]
- Kanski, L.; Naumann, M.; Pawelzik, E. Flavor-related quality attributes of ripe tomatoes are not significantly affected under two common household conditions. Front. Plant Sci. 2020, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- DeVerma, J.W.; Paterson, A.H. Genetics of Lycopersicon. In Genetic Improvement of Tomato; Kalloo, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 3642842771. [Google Scholar]
- Behr, H.-C. AMI Markt Bilanz Gemüse 2019; Agrarmarkt Informations-Gesellschaft mbH: Bonn, Germany, 2019. [Google Scholar]
- Van Heusden, S.; Lindhout, P. Genetics and breeding. In Tomatoes: Crop Production Science in Horticulture, 2nd ed.; Heuvelink, E., Ed.; CAB International: Wallingford, UK, 2018; pp. 27–56. ISBN 9781780641935. [Google Scholar]
- Rocha, M.d.C.; Deliza, R.; Corrêa, F.M.; Carmo, M.G.d.; Abboud, A.C. A study to guide breeding of new cultivars of organic cherry tomato following a consumer-driven approach. Food Res. Int. 2013, 51, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.N.; Hobson, G.E. The constituents of tomato fruit—The influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.P.; Gosselin, A. Greenhouse tomato fruit quality. In Horticultural Reviews; Janick, J., Ed.; Wiley & Sons: New York, NY, USA, 2001; pp. 239–319. ISBN 9780470650806. [Google Scholar]
- Causse, M.; Friguet, C.; Coiret, C.; Lépicier, M.; Navez, B.; Lee, M.; Holthuysen, N.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer preferences for fresh tomato at the European scale: A common segmentation on taste and firmness. J. Food Sci. 2010, 75, S531–S541. [Google Scholar] [CrossRef] [PubMed]
- Statistisches Bundesamt. Area Under Cultivation: Vegetables and Strawberries. Available online: https://www.destatis.de/EN/FactsFigures/EconomicSectors/AgricultureForestryFisheries/FruitVegetablesHorticulture/Tables/1_1Organicfarming.html (accessed on 16 August 2018).
- FiBL. Organic Imports to Germany. Available online: https://www.fibl.org/en/service-en/news-archive/news/article/organic-imports-to-germany.html (accessed on 15 October 2019).
- FAO. World Markets for Organic Fruit and Vegetables: Opportunities for Developing Countries in the Production and Export of Organic Horticultural Products; FAO: Rome, Italy, 2001. [Google Scholar]
- European Council. Commission Regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Control. Available online: http://data.europa.eu/eli/reg/2008/889/2018-01-01 (accessed on 19 September 2018).
- Rauber, R.; Schmidtke, K.; Kimpel-Freund, H. The Performance of Pea (Pisum sativum L.) and its Role in Determining Yield Advantages in Mixed Stands of Pea and Oat (Avena sativa L.). J. Agron. Crop. Sci. 2001, 187, 137–144. [Google Scholar] [CrossRef]
- Erika, C.; Griebel, S.; Naumann, M.; Pawelzik, E. Biodiversity in tomatoes: Is it reflected in nutrient density and nutritional yields under organic outdoor production? Front. Plant Sci. 2020, 11, 589692. [Google Scholar] [CrossRef]
- Liu, J.; Hu, T.; Feng, P.; Wang, L.; Yang, S. Tomato yield and water use efficiency change with various soil moisture and potassium levels during different growth stages. PLoS ONE 2019, 14, e0213643. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Smit, I.; Pawelzik, E.; Keutgen, A.J.; Horneburg, B. Organically grown tomato (Lycopersicon esculentum Mill.): Bioactive compounds in the fruit and infection with Phytophthora infestans. J. Sci. Food Agric. 2012, 92, 1424–1431. [Google Scholar] [CrossRef]
- Capel, C.; Yuste-Lisbona, F.J.; López-Casado, G.; Angosto, T.; Heredia, A.; Cuartero, J.; Fernández-Muñoz, R.; Lozano, R.; Capel, J. QTL mapping of fruit mineral contents provides new chances for molecular breeding of tomato nutritional traits. Theor. Appl. Genet. 2017, 130, 903–913. [Google Scholar] [CrossRef]
- Fess, T.L.; Kotcon, J.B.; Benedito, V.A. Crop breeding for low input agriculture: A sustainable response to feed a growing world population. Sustainability 2011, 3, 1742–1772. [Google Scholar] [CrossRef] [Green Version]
- Zörb, C.; Piepho, H.-P.; Zikeli, S.; Horneburg, B. Heritability and variability of quality parameters of tomatoes in outdoor production. Research 2020, 2020, 6707529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios-Masias, F.H.; Jackson, L.E. California processing tomatoes: Morphological, physiological and phenological traits associated with crop improvement during the last 80 years. Eur. J. Agron. 2014, 53, 45–55. [Google Scholar] [CrossRef]
- Van der Ploeg, A.; van der Meer, M.; Heuvelink, E. Breeding for a more energy efficient greenhouse tomato: Past and future perspectives. Euphytica 2007, 158, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B. Tomato Plant Culture: In the Field, Greenhouse, and Home Garden; CRC Press: Boca Raton, FL, USA; London, UK, 1999; ISBN 0-8493-2025-9. [Google Scholar]
- Higashide, T.; Heuvelink, E. Physiological and morphological changes over the past 50 years in yield components in tomato. J. Amer. Soc. Hort. Sci. 2009, 134, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Van der Ploeg, A.; Heuvelink, E. Influence of sub-optimal temperature on tomato growth and yield: A review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Causse, M.; Saliba-Colombani, V.; Lecomte, L.; Duffe, P.; Rousselle, P.; Buret, M. QTL analysis of fruit quality in fresh market tomato: A few chromosome regions control the variation of sensory and instrumental traits. J. Exp. Bot. 2002, 53, 2089–2098. [Google Scholar] [CrossRef]
- Davis, D.R. Declining fruit and vegetable nutrient composition: What is the evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Piombino, P.; Sinesio, F.; Moneta, E.; Cammareri, M.; Genovese, A.; Lisanti, M.T.; Mogno, M.R.; Peparaio, M.; Termolino, P.; Moio, L.; et al. Investigating physicochemical, volatile and sensory parameters playing a positive or a negative role on tomato liking. Food Res. Int. 2013, 50, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, E. Introduction, definition and classification of nutrients. In Marschner’s Mineral Nutrition of Higher Plant, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Huett, D.O.; Maier, N.A.; Sparrow, L.A.; Piggott, T.J. Vegetable crops. In Plant Analysis: An Interpretation Manual, 2nd ed.; Reuter, D.J., Robinson, J.B., Dutkiewicz, C., Eds.; CSIRO Pub: Colingwood, Australia, 1997; pp. 383–464. ISBN 0643059385. [Google Scholar]
- Nasiyev, B.; Vassilina, T.; Zhylkybay, A.; Shibaikin, V.; Salykova, A. Physicochemical and biological indicators of soils in an organic farming system. Sci. World J. 2021, 2021, 9970957. [Google Scholar] [CrossRef] [PubMed]
- Abduelghader, A.A.; Sanders, F.E.; Pilbeam, D.J. Growth and biomass partitioning in tomato in relation to ratio of nitrogen:phosphorus supply. J. Plant Nutr. 2011, 34, 2018–2038. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.-I.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil Fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamalero, E.; Trotta, A.; Massa, N.; Copetta, A.; Martinotti, M.G.; Berta, G. Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 2004, 14, 185–192. [Google Scholar] [CrossRef]
- Goulding, K.; Stockdale, E.; Watson, A.C. Plant nutrients in organic farming. In Organic Crop Production: Ambitions and Limitations; Kirchmann, H., Bergström, L., Eds.; Springer: Dordrecht, The Netherlands, 2008; ISBN 9781402093159. [Google Scholar]
- Koch, M.; Busse, M.; Naumann, M.; Jákli, B.; Smit, I.; Cakmak, I.; Hermans, C.; Pawelzik, E. Differential effects of varied potassium and magnesium nutrition on production and partitioning of photoassimilates in potato plants. Physiol. Plant. 2019, 166, 921–935. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2019, 10, 1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashinath, B.L.; Murthy, A.N.G.; Senthivel, T.; Pitchai, G.J.; Sadashiva, A.T. Effect of applied magnesium on yield and quality of tomato in Alfisols of Karnataka. J. Hortic. Sci. Biotechnol. 2013, 8, 55–59. [Google Scholar]
- Rosanoff, A. Changing crop magnesium concentrations: Impact on human health. Plant Soil 2012, 368, 139–153. [Google Scholar] [CrossRef]
- Cakmak, I. Magnesium in crop production, food quality and human health. Plant Soil 2013, 368, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Weih, M.; Hamnér, K.; Pourazari, F. Analyzing plant nutrient uptake and utilization efficiencies: Comparison between crops and approaches. Plant Soil 2018, 430, 7–21. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plant, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]




| Cultivar Name | Fruit Colour | Cultivar Name | Fruit Colour |
|---|---|---|---|
| Salad Cultivars (>52 g fruit−1) | Cocktail Cultivars (<52 g fruit−1) | ||
| Previa F1 | Red | Amoroso F1 | Red |
| Garance F1 | Red | Annamay F1 | Red |
| Green Zebra | Green–yellow | Quedlinburger Frühe Liebe | Red |
| Diplom F1 | Red | Ruthje | Red |
| Cappricia F1 | Red | König Humbert | Red |
| Rougella F1 | Red | Clou | Yellow |
| Sparta F1 | Red | Tastery F1 | Red |
| Bocati F1 | Red | Primabella | Red |
| Phantasia F1 | Red | Sakura F1 | Red |
| Mecano F1 | Red | Black Cherry | Red–brown |
| Hamlet F1 | Red | Cerise Gelb | Yellow |
| Lyterno F1 | Red | Yellow Submarine | Yellow |
| Nordica F1 | Red | Zuckertraube | Red |
| Moneymaker | Red | Dorada | Yellow |
| Pannovy F1 | Red | Primavera | Red |
| Roterno F1 | Red | Philovita F1 | Red |
| Hildares F1 | Red | Trixi | Red |
| Bonner Beste | Red | Trilly F1 | Red |
| Tica | Red | Benarys Gartenfreude | Red |
| Ricca | Red | Bartelly F1 | Red |
| Aroma | Red | Golden Pearl F1 | Yellow |
| Rheinlands Ruhm | Red | Resi | Red |
| Lukullus | Red | Supersweet 100 F1 | Red |
| Goldene Königin | Yellow | Goldita | Orange |
| Harzfeuer F1 | Red | Sliwowidnij | Yellow |
| Auriga | Orange | Rote Murmel | Red |
| Haubners Vollendung | Red | Golden Currant | Yellow |
| Dorenia | Red | - | - |
| Roi Humbert Jaune | Yellow | - | - |
| Hellfrucht | Red | - | - |
| Campari F1 | Red | - | - |
| Matina | Red | - | - |
| Black Plum | Red–brown | - | - |
| Cultivar | PH | LN | LSB | FY | AFW | FN | HI | Leaf Minerals (mg g−1 DM) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (Plant−1) | (g Plant−1) | (g Plant−1) | (g Fruit−1) | (Plant−1) | C | N | P | K | Mg | Ca | S | ||
| Salad cultivar (S) | ||||||||||||||
| Green Zebra | 216.3 | 42 | 1520 | 3212 | 145.7 | 22 | 0.76 | 387.1 | 28.1 | 2.0 | 25.8 | 5.2 | 47.8 | 6.0 |
| Cappricia F1 | 284.0 | 49 | 1321 | 5724 | 127.3 | 45 | 0.86 | 382.1 | 24.4 | 1.6 | 22.3 | 3.3 | 59.0 | 7.5 |
| Bocati F1 | 284.4 | 47 | 1408 | 5680 | 119.7 | 48 | 0.85 | 384.0 | 23.9 | 1.7 | 21.5 | 2.9 | 58.8 | 6.8 |
| Lyterno F1 | 308.6 | 51 | 1718 | 5738 | 115.2 | 50 | 0.83 | 383.7 | 23.4 | 1.6 | 23.5 | 3.1 | 56.3 | 6.3 |
| Roterno F1 | 283.8 | 49 | 1286 | 5824 | 105.4 | 56 | 0.86 | 381.8 | 24.3 | 1.5 | 22.4 | 3.5 | 56.4 | 6.8 |
| Harzfeuer F1 | 231.8 | 46 | 1258 | 4031 | 74.1 | 54 | 0.81 | 391.0 | 22.2 | 1.4 | 17.8 | 3.5 | 47.6 | 5.4 |
| Auriga | 223.6 | 45 | 865 | 3498 | 73.8 | 48 | 0.85 | 384.4 | 24.9 | 1.7 | 19.2 | 4.2 | 56.8 | 6.6 |
| Campari F1 | 248.3 | 45 | 707 | 3733 | 62.8 | 59 | 0.87 | 386.1 | 23.4 | 1.6 | 19.8 | 3.3 | 57.1 | 6.7 |
| CV (%) | 13.2 | 5.8 | 26.2 | 24.7 | 28.8 | 23.7 | 4.3 | 0.8 | 7.2 | 11.1 | 11.9 | 8.4 | 20.6 | 9.7 |
| Significance | *** | *** | *** | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | ** |
| HSD (0.05) | 21.6 | 4 | 245 | 568 | 9.8 | 5 | 0.03 | 10.4 | 3.1 | 0.2 | 2.7 | 0.8 | 7.9 | 1.4 |
| Years (Y) | ||||||||||||||
| 2015 | 266.9 | 36 | 1498 | 4611 | 105.2 | 47 | 0.82 | 387.2 | 23.5 | 1.7 | 26.1 | 3.7 | 52.3 | 6.1 |
| 2016 | 254.7 | 52 | 1034 | 4732 | 100.8 | 49 | 0.85 | 382.8 | 25.2 | 1.6 | 17.0 | 3.6 | 57.7 | 6.9 |
| Significance | ** | *** | *** | ns | ** | * | *** | * | ** | *** | *** | ns | ** | *** |
| Interaction (SxY) | ** | * | *** | ns | ns | * | ** | ns | ns | ** | ns | * | ns | ns |
| Cocktail cultivar (C) | ||||||||||||||
| Amoroso F1 | 266.9 | 49 | 1651 | 3539 | 49.3 | 73 | 0.77 | 393.1 | 23.6 | 1.6 | 21.6 | 3.5 | 47.9 | 5.5 |
| Annamay F1 | 303.5 | 53 | 974 | 3902 | 47.1 | 83 | 0.84 | 383.3 | 21.9 | 1.6 | 21.6 | 3.5 | 59.5 | 6.6 |
| Tastery F1 | 307.8 | 51 | 1289 | 3598 | 33.0 | 110 | 0.79 | 394.7 | 26.5 | 1.7 | 25.2 | 3.2 | 48.9 | 6.5 |
| Primabella | 320.0 | 62 | 2583 | 3088 | 27.9 | 111 | 0.62 | 402.9 | 24.8 | 1.9 | 24.7 | 3.1 | 39.4 | 4.5 |
| Sakura F1 | 299.8 | 56 | 822 | 3368 | 23.9 | 141 | 0.84 | 384.0 | 23.0 | 1.5 | 19.6 | 3.3 | 63.2 | 6.0 |
| Black Cherry | 325.9 | 49 | 1394 | 3162 | 24.1 | 133 | 0.76 | 394.4 | 26.2 | 1.7 | 18.9 | 4.4 | 51.6 | 5.2 |
| Primavera | 336.4 | 59 | 1655 | 3838 | 21.4 | 181 | 0.74 | 386.8 | 24.6 | 1.7 | 26.5 | 3.0 | 53.4 | 5.3 |
| Benarys Gartenfreude | 256.1 | 48 | 1048 | 2816 | 18.8 | 150 | 0.78 | 382.7 | 21.0 | 1.5 | 17.4 | 2.9 | 61.7 | 5.2 |
| Bartelly F1 | 321.6 | 53 | 1295 | 4076 | 16.6 | 252 | 0.81 | 386.5 | 25.8 | 1.7 | 25.1 | 4.2 | 58.3 | 6.0 |
| Resi | 346.0 | 59 | 2578 | 1186 | 18.6 | 64 | 0.37 | 398.1 | 25.0 | 1.8 | 21.5 | 4.2 | 40.9 | 5.2 |
| Supersweet 100 F1 | 332.8 | 58 | 1593 | 2905 | 14.7 | 202 | 0.71 | 392.1 | 23.7 | 1.6 | 23.8 | 3.1 | 47.9 | 5.2 |
| Goldita | 225.6 | 53 | 563 | 2115 | 16.3 | 129 | 0.83 | 401.2 | 23.7 | 1.4 | 21.2 | 3.1 | 46.8 | 4.5 |
| CV (%) | 12.0 | 8.4 | 42.9 | 26.2 | 44.8 | 40.7 | 17.8 | 1.8 | 6.9 | 8.7 | 12.6 | 15.1 | 14.8 | 12.5 |
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| HSD (0.05) | 30.1 | 5 | 417 | 485 | 2.9 | 25 | 0.05 | 8.5 | 3.2 | 0.2 | 4.1 | 0.7 | 7.9 | 1.1 |
| Years (Y) | ||||||||||||||
| 2015 | 297.8 | 42 | 1621 | 2938 | 25.6 | 127 | 0.72 | 387.0 | 23.6 | 1.7 | 26.4 | 3.9 | 55.2 | 5.6 |
| 2016 | 308.9 | 60 | 1287 | 3323 | 25.9 | 146 | 0.76 | 396.3 | 24.8 | 1.6 | 18.1 | 3.0 | 48.1 | 5.3 |
| Significance | ** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** | *** | * |
| Interaction (CxY) | *** | *** | *** | *** | *** | *** | *** | ns | *** | ns | * | ns | ns | ns |
| Cultivar | Fruit Minerals (mg 100 g−1 FW) | DM | TSS | TA | TPC | Fruit Colour | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P # | K # | Mg # | Ca # | S | (%) | (°Brix) | (%) | (mg GAE 100 g−1) FW) | L * | a * | b * | C * | °h | |
| Salad cultivar (S) | ||||||||||||||
| Green Zebra | 30.7 | 297.4 | 14.2 | 13.2 | 15.6 | 5.7 | 5.1 | 0.65 | 139.2 | 58.9 | −10.7 | 37.0 | 41.9 | 105.4 |
| Cappricia F1 | 25.9 | 246.9 | 8.5 | 15.3 | 13.0 | 5.6 | 4.6 | 0.35 | 109.0 | 53.4 | 22.5 | 28.4 | 39.6 | 54.0 |
| Bocati F1 | 21.4 | 212.4 | 8.3 | 12.4 | 11.0 | 5.2 | 5.1 | 0.38 | 105.2 | 53.1 | 24.4 | 28.0 | 40.1 | 51.2 |
| Lyterno F1 | 26.2 | 243.7 | 9.2 | 15.2 | 13.5 | 5.9 | 4.6 | 0.38 | 131.8 | 52.8 | 20.0 | 25.6 | 35.8 | 54.4 |
| Roterno F1 | 24.4 | 237.9 | 9.4 | 14.5 | 13.2 | 5.5 | 4.5 | 0.37 | 119.3 | 53.3 | 21.6 | 27.8 | 38.3 | 54.4 |
| Harzfeuer F1 | 26.8 | 303.2 | 10.9 | 11.7 | 14.4 | 6.8 | 5.5 | 0.42 | 143.4 | 50.4 | 19.5 | 23.8 | 34.1 | 53.4 |
| Auriga | 29.0 | 299.0 | 13.5 | 8.0 | 14.7 | 6.9 | 5.5 | 0.53 | 160.8 | 61.5 | 13.5 | 42.8 | 47.5 | 73.1 |
| Campari F1 | 30.1 | 293.9 | 11.9 | 13.7 | 15.0 | 6.9 | 6.1 | 0.47 | 158.1 | 50.2 | 17.0 | 23.1 | 31.9 | 56.4 |
| CV (%) | 11.6 | 13.3 | 18.5 | 21.2 | 10.6 | 11.6 | 10.6 | 23.4 | 15.7 | 7.3 | 70.6 | 23.2 | 12.6 | 29.5 |
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| HSD (0.05) | 5.8 | 56.5 | 2.2 | 4.6 | 2.8 | 1.1 | 1.0 | 0.08 | 21.7 | 1.9 | 2.0 | 2.8 | 3.3 | 6.4 |
| Years (Y) | ||||||||||||||
| 2015 | 28.7 | 280.1 | 10.6 | 13.2 | 14.9 | 5.8 | 4.7 | 0.43 | 177.0 | 54.1 | 18.2 | 30.4 | 37.1 | 58.8 |
| 2016 | 25.0 | 254.1 | 10.9 | 12.8 | 12.8 | 6.3 | 5.5 | 0.46 | 93.0 | 54.2 | 14.5 | 28.6 | 39.9 | 65.5 |
| Significance | *** | ** | ns | ns | *** | * | *** | ns | *** | ns | *** | ** | ns | ** |
| Interaction (SxY) | ns | ns | ns | ** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Cocktail cultivar (C) | ||||||||||||||
| Amoroso F1 | 27.8 | 233.8 | 9.8 | 12.9 | 13.9 | 7.4 | 6.0 | 0.49 | 151.7 | 49.6 | 13.3 | 20.4 | 27.9 | 59.6 |
| Annamay F1 | 31.3 | 314.2 | 13.4 | 15.0 | 15.4 | 7.1 | 6.2 | 0.50 | 176.8 | 50.8 | 17.3 | 22.7 | 32.0 | 54.9 |
| Tastery F1 | 31.5 | 266.9 | 11.6 | 13.7 | 15.1 | 8.3 | 6.7 | 0.39 | 167.8 | 51.4 | 13.1 | 23.1 | 30.1 | 62.5 |
| Primabella | 38.5 | 339.3 | 14.4 | 13.4 | 19.1 | 8.3 | 6.8 | 0.54 | 203.1 | 50.4 | 19.9 | 23.1 | 33.7 | 51.6 |
| Sakura F1 | 31.3 | 297.6 | 12.9 | 12.4 | 14.6 | 9.0 | 7.7 | 0.53 | 200.7 | 49.3 | 13.2 | 21.3 | 28.5 | 60.1 |
| Black Cherry | 27.4 | 253.8 | 11.6 | 11.9 | 14.9 | 7.6 | 6.9 | 0.53 | 172.2 | 47.9 | 4.8 | 9.8 | 16.1 | 66.5 |
| Primavera | 30.5 | 273.2 | 10.6 | 13.1 | 16.4 | 7.4 | 6.2 | 0.38 | 188.4 | 49.6 | 12.5 | 21.0 | 28.2 | 61.5 |
| Benarys Gartenfreude | 36.6 | 334.3 | 12.8 | 9.6 | 18.0 | 10.4 | 8.3 | 0.55 | 177.6 | 50.0 | 13.9 | 20.5 | 28.5 | 58.6 |
| Bartelly F1 | 39.0 | 320.9 | 13.5 | 16.9 | 18.8 | 8.5 | 7.7 | 0.45 | 211.8 | 49.9 | 14.0 | 20.2 | 28.2 | 57.6 |
| Resi | 48.2 | 360.6 | 16.6 | 19.4 | 21.7 | 8.0 | 6.9 | 0.47 | 205.4 | 50.5 | 18.5 | 20.9 | 31.6 | 51.0 |
| Supersweet 100 F1 | 42.7 | 344.8 | 15.5 | 9.7 | 19.9 | 10.1 | 8.2 | 0.54 | 224.2 | 49.7 | 15.9 | 21.4 | 30.1 | 55.7 |
| Goldita | 33.6 | 310.6 | 13.2 | 17.7 | 20.1 | 9.5 | 7.4 | 0.51 | 275.6 | 56.2 | 7.0 | 31.3 | 35.5 | 78.3 |
| CV (%) | 18.1 | 13.0 | 21.7 | 14.9 | 15.0 | 12.7 | 10.9 | 11.6 | 16.6 | 4.0 | 31.7 | 22.0 | 16.4 | 12.2 |
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| HSD (0.05) | 8.4 | 70.7 | 3.3 | 5.4 | 4.6 | 1.7 | 0.6 | 0.08 | 33.0 | 1.2 | 2.6 | 2.3 | 3.4 | 5.9 |
| Years (Y) | ||||||||||||||
| 2015 | 35.0 | 297.5 | 12.2 | 14.3 | 17.5 | 8.3 | 6.6 | 0.45 | 250.3 | 50.2 | 14.5 | 22.0 | 26.8 | 56.3 |
| 2016 | 34.7 | 310.9 | 13.8 | 13.4 | 17.2 | 8.7 | 7.6 | 0.53 | 142.3 | 50.7 | 12.7 | 20.6 | 31.6 | 63.4 |
| Significance | ns | *** | *** | ns | ns | ns | *** | *** | *** | ** | *** | *** | * | *** |
| Interaction (CxY) | * | ** | ** | * | * | ns | ns | ns | ** | *** | *** | *** | ns | ns |
| Cultivar | LSB | Fruit Yield | Leaf N | Leaf P | Leaf Mg | Fruit Mg | Fruit TSS | Fruit TA | Fruit TPC |
|---|---|---|---|---|---|---|---|---|---|
| Salad cultivars | |||||||||
| Lyterno F1 | X | ||||||||
| Green Zebra | X | X | X | X | X | X | |||
| Roterno F1 | X | ||||||||
| Auriga F1 | X | X | X | X | X | ||||
| Campari F1 | X | ||||||||
| Cocktail cultivars | |||||||||
| Tastery F1 | X | X | |||||||
| Primabella | X | X | X | X | X | X | |||
| Bartelly F1 | X | ||||||||
| Resi | X | X | X | X | X | ||||
| Supersweet 100 F1 | X | X | X | X | |||||
| Goldita | X | X | X | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chea, L.; Erika, C.; Naumann, M.; Smit, I.; Horneburg, B.; Pawelzik, E. Morphological, Leaf Nutrient, and Fruit Quality Characteristics of Diverse Tomato Cultivars under Organic Low-Input Management. Sustainability 2021, 13, 12326. https://doi.org/10.3390/su132112326
Chea L, Erika C, Naumann M, Smit I, Horneburg B, Pawelzik E. Morphological, Leaf Nutrient, and Fruit Quality Characteristics of Diverse Tomato Cultivars under Organic Low-Input Management. Sustainability. 2021; 13(21):12326. https://doi.org/10.3390/su132112326
Chicago/Turabian StyleChea, Leangsrun, Cut Erika, Marcel Naumann, Inga Smit, Bernd Horneburg, and Elke Pawelzik. 2021. "Morphological, Leaf Nutrient, and Fruit Quality Characteristics of Diverse Tomato Cultivars under Organic Low-Input Management" Sustainability 13, no. 21: 12326. https://doi.org/10.3390/su132112326