Improving the Efficiency of Lambari Production and Diet Assimilation Using Integrated Aquaculture with Benthic Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Feeding Management
2.3. Water Quality
2.4. Harvest and Productivity Data Collection
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Global Aquaculture Production. FAO Fisheries and Aquaculture Statistics; FAO: Rome, Italy, 2021; Available online: http://www.fao.org/figis/servlet/SQServlet?file=/usr/local/tomcat/8.5.16/figis/webapps/figis/temp/hqp_4125957264323673027.xml&outtype=html (accessed on 29 July 2021).
- FAO. The State of World Fisheries and Aquaculture 2020; Sustainability in action; FAO: Rome, Italy, 2020. [Google Scholar]
- Béné, C.; Barange, M.; Subasinghe, R.; Pinstrup-Andersen, P.; Merino, G.; Hemre, G.I.; Williams, M. Feeding 9 billion by 2050–Putting fish back on the menu. Food Secur. 2015, 7, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Hardy, R.W.; Gatlin, D. Nutritional strategies to reduce nutrient losses in intensive aquaculture. In Avances en Nutrición Acuícola VI. Proceedings of the VI Simposium Internacional de Nutrición Acuícola, Cancun, Quintana Roo, Mexico, 3–6 September 2002; Cruz-Suárez, L.E., Ricque-Marie, D., Tapia-Salazar, M., Gaxiola-Cortés, M.G., Simoes, N., Eds.; Universidad Autónoma de Nuevo León: Monerrey, Mexico, 2002. [Google Scholar]
- Tacon, A.G.J.; Forster, I.P. Aquafeeds and the environment: Policy implications. Aquaculture 2003, 226, 181–189. [Google Scholar] [CrossRef]
- Mongirdas, V.; Žibienė, G.; Žibas, A. Waste and its characterization in closed recirculating aquaculture systems—A review. J. Water Secur. 2017, 3, jws2017002. [Google Scholar] [CrossRef] [Green Version]
- Henares, M.N.; Medeiros, M.V.; Camargo, A.F. Overview of strategies that contribute to the environmental sustainability of pond aquaculture: Rearing systems, residue treatment, and environmental assessment tools. Rev. Aquac. 2020, 12, 453–470. [Google Scholar] [CrossRef]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When more is more: Taking advantage of species diversity to move towards sustainable aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef]
- Chopin, T.; MacDonald, B.; Robinson, S.; Cross, S.; Pearce, C.; Knowler, D.; Noce, A.; Reid, G.; Cooper, A.; Speare, D.; et al. The Canadian Integrated Multi-Trophic Aquaculture Network (CIMTAN)—A Network for a New Era of Ecosystem Responsible Aquaculture. Fisheries 2013, 38, 297–308. [Google Scholar] [CrossRef]
- Henriques, M.B.; Fagundes, L.; Pretesse, M.L.; Silva, N.J.R.; Rezende, K.F.O.; Barbieri, E. Lambari fish Deuterodon iguape as an alternative to live bait for estuarine recreational fishing. Fish. Manag. Ecol. 2018, 25, 400–407. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- Lucena, C.A.; Soares, H.G. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La plata and rio São Francisco drainages and coastal system of southern Brazil and Uruguay. Zootaxa 2016, 4072, 101–125. [Google Scholar]
- Ferreira, P.M.F.; Nascimento, L.S.; Dias, D.C.; Moreira, D.M.V.; Salaro, A.L.; Freitas, M.B.D.; Carneiro, A.P.S.; Zuanon, J.A.S. Essential oregano oil as a growth promoter for the yellow tail tetra, Astyanax altiparanae. J. World Aquac. Soc. 2014, 45, 28–34. [Google Scholar] [CrossRef]
- Fonseca, T.; Costa-Pierce, B.A.; Valenti, W.C. Lambari Aquaculture as a Means for the Sustainable Development of Rural Communities in Brazil. Rev. Fish. Sci. Aquac. 2017, 25, 316–330. [Google Scholar] [CrossRef]
- Kensley, B.; Walker, I. Palaemonid Shrimps from the Amazon Basin, Brazil (Crustacea: Decapoda: Natantia). Smithson. Contrib. Zool. 1982, 362, 1–27. [Google Scholar] [CrossRef] [Green Version]
- David, F.S.; Proença, D.C.; Valenti, W.C. Phosphorus budget in integrated multitrophic aquaculture systems with Nile Tilapia, Oreochromis niloticus, and Amazon River prawn, Macrobrachium amazonicum. J. World Aquac. Soc. 2017, 48, 402–414. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Valenti, W.C. Nitrogen budget in integrated aquaculture systems with Nile tilapia and Amazon River prawn. Aquac. Int. 2017, 25, 1733–1746. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Flickinger, D.L.; Bueno, G.W.; Valenti, W.C. Carbon budget in integrated aquaculture systems with Nile tilapia (Oreochromis niloticus) and Amazon river prawn (Macrobrachium amazonicum). Aquac. Res. 2021, 52, 1–13. [Google Scholar]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Moraes-Valenti, P.; Valenti, W.C. The budget of nitrogen in the grow-out of the Amazon river prawn (Macrobrachium amazonicum Heller) and tambaqui (Colossoma macropomum Cuvier) farmed in monoculture and in integrated multitrophic aquaculture systems. Aquac. Res. 2019, 50, 3444–3461. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Dantas, D.P.; Proença, D.C.; David, F.S.; Valenti, W.C. Phosphorus in the culture of the Amazon river prawn (Macrobrachium amazonicum) and tambaqui (Colossoma macropomum) farmed in monoculture and in integrated multitrophic systems. J. World Aquac. Soc. 2020, 51, 1002–1023. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Proença, D.C.; David, F.S.; Durborow, R.M.; Moraes-Valenti, P.; Valenti, W.C. The budget of carbon in the farming of the Amazon river prawn and tambaqui fish in earthen pond monoculture and integrated multitrophic systems. Aquac. Rep. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Wright, J.P.; Flecker, A.S. Deforesting the riverscape: The effects of wood on fish in a Venezuelan piedmont stream. Biol. Conserv. 2004, 120, 439–447. [Google Scholar] [CrossRef]
- Taylor, B.W.; Flecker, A.S.; Hall, R.O., Jr. Loss of a harvested fish species disrupts carbon flow in a diverse tropical river. Science 2006, 313, 833–836. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, L.A.; Ono, E.; Routledge, E.A.B.; Correia, E.S.; Moraes-Valenti, P.; Martino, R.C. Brazilian aquaculture update. World Aquacultulture 2010, 35, 41–68. [Google Scholar]
- Freire, K.M.F.; Machado, M.L.; Crepaldi, D. Overview of inland recreational fisheries in Brazil. Fisheries 2012, 37, 484–494. [Google Scholar] [CrossRef]
- Saint-Paul, U. Native fish species boosting Brazilian’s aquaculture development. Acta Fish. Aquat. Resour. 2017, 5, 1–9. [Google Scholar]
- Fugi, R.; Hahn, N.S.; Agostinho, A.A. Feeding styles of five species of bottom-feeding fishes of the high Paraná River. Environ. Biol. Fishes 1996, 46, 297–307. [Google Scholar] [CrossRef]
- Kalous, L.; Bui, A.T.; Petrtyl, M.; Bohlen, J.; Chaloupkova, P. The south American freshwater fish Prochilodus lineatus (Actinopterygii: Characiformes: Prochilodontidae): New species in Vietnamese aquaculture. Aquac. Res. 2012, 43, 955–958. [Google Scholar] [CrossRef]
- D’Abramo, L.R.; Sheen, S.S. Nutritional requirements, feed formulation, and feeding practices for intensive culture of the freshwater prawn Macrobrachium rosenbergii. Rev. Fish. Sci. 1994, 2, 1–21. [Google Scholar] [CrossRef]
- Franchini, A.C.; Costa, G.A.; Pereira, S.A.; Valenti, W.C.; Moraes-Valenti, P. Improving production and diet assimilation in fish-prawn integrated aquaculture, using iliophagus species. Aquaculture 2020, 521, 735048. [Google Scholar] [CrossRef]
- Dantas, D.P.; Flickinger, D.L.; Costa, G.A.; Batlouni, S.R.; Moraes-Valenti, P.; Valenti, W.C. Technical feasibility of integrating Amazon river prawn culture during the first phase of tambaqui grow-out in stagnant ponds, using nutrient-rich water. Aquaculture 2020, 516, 734611. [Google Scholar] [CrossRef]
- Boyd, C.E. Water Quality: An Introduction; Springer Nature: Alburn, AL, USA, 2019. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA: Washington, DC, USA, 2017. [Google Scholar]
- Brown, T.; Simpson, J. Managing phosphorus inputs to urban lakes: I. Determining the trophic state of your lake. Watershed Prot. Tech. 2001, 3, 771. [Google Scholar]
- Rodrigues, C.G.; Garcia, B.F.; Verdegem, M.; Santos, M.R.; Amorim, R.V.; Valenti, W.C. Integrated culture of Nile tilapia and Amazon river prawn in stagnant ponds, using nutrient-rich water and substrates. Aquaculture 2019, 503, 111–117. [Google Scholar] [CrossRef]
- Vilela, C.; Hayashi, C. Desenvolvimento de juvenis de lambari Astyanax bimaculatus (Linnaeus, 1758), sob diferentes densidades de estocagem em tanques-rede. Acta Scientiarum. Biol. Sci. 2001, 23, 491–496. [Google Scholar]
- Henriques, M.B.; Caeneiro, J.S.; Fagundes, L.; Castilho-Barros, L.; Barbieri, E. Economic feasibility for the production of live baits of lambari (Deuterodon iguape) in recirculations system. Bol. Inst. Pesca 2019, 45, 516–524. [Google Scholar] [CrossRef]
- Marques, H.L.A.; Moraes-Valenti, P. Current status and prospects of farming the giant river prawn Macrobrachium rosenbergii (De M an 1879) and the Amazon river prawn Macrobrachium amazonicum (Heller 1862)) in Brazil. Aquac. Res. 2012, 43, 984–992. [Google Scholar] [CrossRef]
- Moraes-Valenti, P.; Valenti, W.C. Effect of intensification on grow out of the Amazon River prawn, Macrobrachium amazonicum. J. World Aquac. Soc. 2007, 38, 516–526. [Google Scholar] [CrossRef]
- Kimpara, J.M.; Rosa, F.R.T.; Preto, B.L.; Valenti, W.C. Limnology of Macrobrachium amazonicum grow-out ponds subject to high inflow of nutriente-rich water and diferente stocking and harvest management. Aquac. Res. 2011, 42, 1289–1297. [Google Scholar] [CrossRef]
- Ibrahim, A.N.A.F.; Karplus, I.; Valenti, W.C. Social interaction in males of the Amazon river prawn Macrobrachium amazonicum (Heller, 1862) (Decapoda, Palaemonidae). Crustaceana 2021, 94, 325–341. [Google Scholar] [CrossRef]
- Fugi, R.; Agostinho, A.A.; Hahn, N.S. Trophic morphology of five benthic feeding fish species of a tropical floodplain. Rev. Bras. Biol. 2001, 61, 27–33. [Google Scholar] [CrossRef]
- Marques, H.L.A.; New, M.B.; Boock, M.V.; Barros, H.P.; Mallasen, M.; Valenti, W.C. Integrated freshwater prawn farming: State-of-the-art and future potential. Rev. Fish. Sci. Aquac. 2016, 24, 264–293. [Google Scholar] [CrossRef]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.L.; Moraes-Valenti, P. Indicators of sustainability to assess aquaculture systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American fish for continental aquaculture. Rev. Aquac. 2018, 10, 351–369. [Google Scholar] [CrossRef]

| Variables | Treatment | p * | |||
|---|---|---|---|---|---|
| LM | LP | LPC | |||
| Temperature | °C | 27.6 ± 0.2 | 27.9 ± 0.2 | 27.9 ± 0.2 | 0.252 |
| DO | mg L−1 | 4.8 ± 0.3 b | 6.0 ± 0.5 a | 5.4 ± 0.2 b | 0.006 |
| pH | - | 8.0 ± 0.1 | 8.1 ± 0.4 | 8.1 ± 0.2 | 0.546 |
| Conductivity | µS cm−1 | 136 ± 1 | 134 ± 1 | 136 ± 1 | 0.162 |
| TSS | mg L−1 | 17.2 ± 8.1 | 15.7 ± 4.9 | 14.9 ± 5.2 | 0.768 |
| Nitrogen | µg L−1 | 425 ± 186 | 477 ± 135 | 462 ± 136 | 0.554 |
| Phosphorous | µg L−1 | 164 ± 54 | 192 ± 58 | 173 ± 52 | 0.216 |
| LM | LP | LPC | |
|---|---|---|---|
| Total yield (t ha−1 cycle−1) | 1.8 ± 0.4 c | 2.4 ± 0.2 b | 3.2 ± 0.5 a |
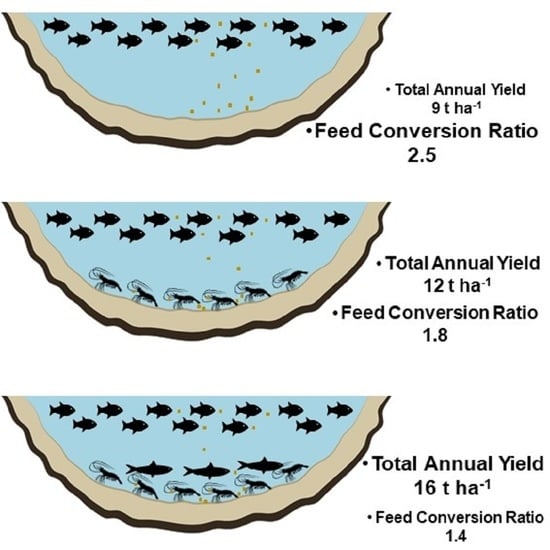
| Total anual yield (t ha−1) | 9 | 12 | 16 |
| Total FCR | 2.5 ± 0.8 a | 1.8 ± 0.3 ab | 1.4 ± 0.3 b |
| Astyanax lacustris | |||
| FCR | 2.5 ± 0.8 | 2.2 ± 0.3 | 2.0 ± 0.6 |
| Mean final mass (g) | 7.7 ± 0.8 | 7.8 ± 0.9 | 7.7 ± 0.8 |
| Mean final length (cm) | 7.3 ± 0.2 | 7.4 ± 0.3 | 7.4 ± 0.1 |
| Survival (%) | 46 ± 8 | 48 ± 9 | 56 ± 14 |
| Yield (t ha−1) | 1.8 ± 0.4 | 1.8 ± 0.2 | 2.1 ± 0.5 |
| Macrobrachium amazonicum | |||
| Mean final mass (g) | - | 2.7 ± 0.2 | 2.4 ± 0.2 |
| Mean final length (cm) | - | 6.8 ± 0.2 | 6.6 ± 0.2 |
| Survival (%) | - | 80 ± 4 | 81 ± 3 |
| Yield (t ha−1) | - | 0.6 ± 0.1 | 0.5 ± 0.0 |
| Prochilodus lineatus | |||
| Mean final mass (g) | - | - | 6.1 ± 2.2 |
| Mean final length (cm) | - | - | 7.3 ± 0.7 |
| Survival (%) | - | - | 70 ± 10 |
| Yield (t ha−1) | - | - | 0.5 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.M.; Boaratti, A.Z.; Belmudes, D.; Ferreira, J.R.C.; Mantoan, P.V.L.; Moraes-Valenti, P.; Valenti, W.C. Improving the Efficiency of Lambari Production and Diet Assimilation Using Integrated Aquaculture with Benthic Species. Sustainability 2021, 13, 10196. https://doi.org/10.3390/su131810196
Marques AM, Boaratti AZ, Belmudes D, Ferreira JRC, Mantoan PVL, Moraes-Valenti P, Valenti WC. Improving the Efficiency of Lambari Production and Diet Assimilation Using Integrated Aquaculture with Benthic Species. Sustainability. 2021; 13(18):10196. https://doi.org/10.3390/su131810196
Chicago/Turabian StyleMarques, Aline M., Andre Z. Boaratti, Dalton Belmudes, Julia R. C. Ferreira, Paulo V. L. Mantoan, Patricia Moraes-Valenti, and Wagner C. Valenti. 2021. "Improving the Efficiency of Lambari Production and Diet Assimilation Using Integrated Aquaculture with Benthic Species" Sustainability 13, no. 18: 10196. https://doi.org/10.3390/su131810196