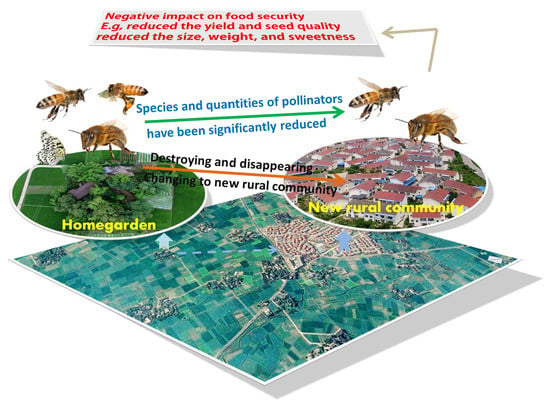
Pollination Services from Insects in Homegardens in the Chengdu Plain will be Confronted with Crises
Abstract
:1. Introduction
2. Study Area
3. Methods and Data Analysis
3.1. Insect Pollination Experiment
3.2. Crop Pollination Dependence ()
3.3. Distribution and Number of Managed Bees
3.4. Changes in Insect Habitat Patterns
3.5. Data Analysis
4. Results
4.1. Pollinator Species and Number of Flower-Visits
4.2. Agricultural Crop Yield and Quality
4.3. Crop Pollination Dependence ()
4.4. Survey of Managed Bees
5. Discussion
5.1. Result Accuracy and Error
5.2. The Importance and Uniqueness of Insect Pollination Services
5.3. The Main Challenges to Insect Pollination in the Chengdu Plain
5.4. Insect Pollination Services Improvement Plan
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoehn, P.; Tscharntke, T.; Tylianakis, J.M.; Dewenter, I.S. Functional group diversity of bee pollinators increases crop yield. Proc. Boil. Sci. 2008, 275, 2283–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfree, R.; Gross, B.J.; Kremen, C. Valuing pollination services to agriculture. Ecol. Econ. 2011, 71, 80–88. [Google Scholar] [CrossRef]
- Board, M.E.A. Ecosystems and Human Well-being: Synthesis. Phys. Teach. 2005, 34, 534. [Google Scholar]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. Boil. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and Temporal Trends of Global Pollination Benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Garratt, M.P.D.; Breeze, T.D.; Jenner, N.; Polce, C.; Biesmeijer, J.C.; Potts, S.G. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 2014, 184, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Boil. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Gonzálezvaro, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szentgyrgyi, H.; Woyciechowski, M.; Vilà, M. Combined effects of global change pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Deguines, N.; Jono, C.; Baude, M.; Henry, M.; Julliard, R.; Fontaine, C. Large-scale trade-off between agricultural intensification and crop pollination services. Front. Ecol. Environ. 2016, 12, 212–217. [Google Scholar] [CrossRef]
- Vanbergen, A.J. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Albrecht, M.; Duelli, P.; Muller, C.; Kleijn, D.; Schmid, B. The Swiss agri-environment scheme enhances pollinator diversity and plant reproductive success in nearby intensively managed farmland. J. Appl. Ecol. 2007, 44, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Brittain, C.; Hendrix, S.D.; Thorp, R.; Williams, N.; Kremen, C. Wild pollination services to California almond rely on semi-natural habitat. J. Appl. Ecol. 2012, 49, 723–732. [Google Scholar] [CrossRef]
- Brosi, B.J.; Armsworth, P.R.; Daily, G.C. Optimal design of agricultural landscapes for pollination services. Conserv. Lett. 2008, 1, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Hannon, L.E.; Sisk, T.D. Hedgerows in an agri-natural landscape: Potential habitat value for native bees. Boil. Conserv. 2009, 142, 2140–2154. [Google Scholar] [CrossRef]
- Korpela, E.L.; Hyvönen, T.; Lindgren, S.; Kuussaari, M. Can pollination services, species diversity and conservation be simultaneously promoted by sown wildflower strips on farmland? Agric. Ecosyst. Environ. 2013, 179, 18–24. [Google Scholar] [CrossRef]
- Kremen, C.; Miles, A. Ecosystem Services in Biologically Diversified versus Conventional Farming Systems: Benefits, Externalities, and Trade-Offs. Ecol. Soc. 2012, 17, 388–395. [Google Scholar] [CrossRef]
- Roschewitz, I.; Gabriel, D.; Tscharntke, T.; Thies, C. The effects of landscape complexity on arable weed species diversity in organic and conventional farming. J. Appl. Ecol. 2005, 42, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Rossum, F.V. Reproductive success and pollen dispersal in urban populations of an insect-pollinated hay-meadow herb. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 21–29. [Google Scholar] [CrossRef]
- Marshall, E.; Moonen, A.C. Field margins in northern Europe: Their functions and interactions with agriculture. Agric. Ecosyst. Environ. 2002, 89, 5–21. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef] [Green Version]
- Winfree, R.; Kremen, C. Are ecosystem services stabilized by differences among species? A test using crop pollination. Proc. Boil. Sci. 2009, 276, 229–237. [Google Scholar] [CrossRef]
- Ricou, C.; Schneller, C.; Amiaud, B.; Plantureux, S.; Bockstaller, C. A vegetation-based indicator to assess the pollination value of field margin flora. Ecol. Indic. 2014, 45, 320–331. [Google Scholar] [CrossRef]
- Freitas, B.M.; Imperatrizfonseca, V.L.; Medina, L.M.; Kleinert, A.M.P.; Galetto, L.; Parra, G.N.; Quezada-Euán, J.J.G. Diversity, threats and conservation of native bees in the Neotropics. Apidologie 2009, 40, 332–346. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, D.M.; Matteson, K.C.; Minor, E.S. Diversity of wild bees supports pollination services in an urbanized landscape. Oecologia 2015, 179, 811–821. [Google Scholar] [CrossRef]
- Li, H.Y. Economic Value of Bee Pollination in China. Ph.D. Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2012. [Google Scholar]
- Guedes, J.D.A. Millets, Rice, Social Complexity, and the Spread of Agriculture to the Chengdu Plain and Southwest China. Rice 2011, 4, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.R.; Li, X.K. On Causes for Formation of Linpan culture in West Sichuan Plain. J. Chengdu Univ. 2011, 5, 45–49. [Google Scholar]
- Li, Y.F.; Zhan, J.Y.; Liu, Y.; Zhang, F.; Zhang, M.L. Response of ecosystem services to land use and cover change: A case study in Chengdu City. Resour. Conserv. Recycl. 2017, 132, 291–300. [Google Scholar] [CrossRef]
- Yang, Q.J.; Li, B.; Li, K. The Rural Landscape Research in Chengdu’s Urban-rural Intergration Development. Procedia Eng. 2011, 21, 780–788. [Google Scholar]
- Liu, Q.; Wang, Y.K.; Guo, Y.M.; Peng, P.H.; Wang, K.Y. Morphological characteristics and composition of plant species and their distribution patterns in Linpan of Chengdu plain. Acta Ecol. Sin. 2018, 38, 3553–3561. [Google Scholar]
- Nair, P.K.R. Do tropical homegardens elude science, or is it the other way around? Agrofor. Syst. 2001, 53, 239–245. [Google Scholar] [CrossRef]
- Trinh, L.N.; Watson, J.W.; Hue, N.N.; De, N.N.; Minh, N.V.; Chu, P.; Sthapit, B.R.; Eyzaguirre, P.B. Agrobiodiversity conservation and development in Vietnamese home gardens. Agric. Ecosyst. Environment. 2003, 97, 317–344. [Google Scholar] [CrossRef]
- Kabir, M.E.; Webb, E.L. Household and homegarden characteristics in southwestern Bangladesh. Agrofor. Syst. 2009, 75, 129–145. [Google Scholar] [CrossRef]
- Guo, Y.M.; Xu, P.; Liu, Q.; Wang, K.Y.; Wang, H.W. Spatial Distribution Characteristics of Linpan in Chengdu Plain—A Case of Pi County. J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2017, 42, 121–126. [Google Scholar]
- Luo, Z.; Yang, C.J.; Li, X.W. Extracting the Rural Residential Information from High Resolution Satellite Imagery. Geospat. Inf. 2009, 7, 80–82. [Google Scholar]
- Shu, B. Research on the Agriculture Landscape of Cheng Plain. Ph.D. Thesis, Southwest Jiaotong University, Chengdu, China, 2011. [Google Scholar]
- Koohafkan, P.; Altieri, M.A. Globally Important Agricultural Heritage Systems (GIAHS). A Legacy for the Future; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Nath, A.J.; Das, A.K. Carbon storage and sequestration in bamboo-based smallholder homegardens of Barak Valley, Assam. Curr. Sci. 2011, 100, 229–233. [Google Scholar]
- Galhena, D.H.; Freed, R.; Maredia, K.M. Home gardens: A promising approach to enhance household food security and wellbeing. Agric. Food Secur. 2013, 2, 1–13. [Google Scholar] [CrossRef]
- Mohri, H.; Lahoti, S.; Saito, O.; Mahalingam, A.; Gunatilleke, N.; Hitinayake, G.; Takeuchi, K.; Herath, S. Assessment of ecosystem services in homegarden systems in Indonesia, Sri Lanka, and Vietnam. Ecosyst. Serv. 2013, 5, 124–136. [Google Scholar] [CrossRef]
- Karthigesu, J.; Sivachndiran, S.; Pushpakumara, D.; Weerahewa, J. Ecosystem Services of Homegarden Agroforestry in Jaffna Peninsula. Int. Conf. Dry Zone Agric. 2016, 3, 56–68. [Google Scholar]
- Zhou, Y.; Chen, J. Landscape Pattern Change and Its Driving Forces in the Linpan of Western Sichuan. J. Sichuan Agric. Univ. 2017, 35, 241–250. [Google Scholar]
- Guo, Y.M. Study on Spatial-TemporalVariation Characteristics and Driving Forces of Linpan in Chengdu Plain—A Case of Pi County. Master. Thesis, University of Chinese Academy of Science, Beijing, China, 2017. [Google Scholar]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. R. Soc. B Boil. Sci. 2014, 281, 20132440. [Google Scholar] [CrossRef]
- Luo, W.H.; Dai, R.G.; Wang, R.S.; Cao, L.; Guo, J.; Ren, Q.; Ji, C.H.; Cheng, S. Study on the effect of insect pollination on rapeseed in Chongqing area. Heilongjiang Anim. Sci. 2014, 11, 120–121. [Google Scholar]
- Holzschuh, A.; Dudenhöffer, J.H.; Tscharntke, T. Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Boil. Conserv. 2012, 153, 101–107. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Kremen, C.; Morales, J.M.; Bommarco, R.; Cunningham, S.A.; Carvalheiro, L.G.; Chacoff, N.P.; Dudenhffer, J.H.; Greenleaf, S.S.; et al. Stability of pollination services decreases with isolation from natural areas despite honey bee visits. Ecol. Lett. 2011, 14, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- He, J.H.; Li, Y.L.; Yang, Y.J.; Zhao, J.; Wu, T.; Wu, Z.X.; Zhao, G.; Dong, K.; He, S.Y. Study on The Effects of Pollination by Honey-Bees on Peach Tress. J. Bee 2011, 30, 5–6. [Google Scholar]
- Shi, Y.Y.; Guan, C.; Zeng, Z.J.; An, J.D.; Luo, S.D. Yield—Increasing Effect and Mechanism of Honey bee on Rape Pollination. Acta Agric. Univ. Jiangxiensis 2009, 31, 994–999. [Google Scholar]
- Liu, P.F.; Wu, J.; Li, H.Y.; Lin, S.W. Economic Values of Bee Pollination to China’s Agriculture. Sci. Agric. Sin. 2011, 44, 5117–5123. [Google Scholar]
- An, D.J.; Chen, W.F. Economic value of insect pollination for fruits and vegetables in China. Acta Èntomol. Sin. 2011, 54, 443–450. [Google Scholar]
- Zhong, Y.H.; Zhao, D.X.; Gao, J.L.; Wang, Y.J.; Liu, J.F. Study of Pollinating Insect of Dimocarpus longana. Apic. China. 2014, 65, 61–63. [Google Scholar]
- Li, Y.H.; Xiang, B.; Yuan, X.Z.; Xie, Q.; Li, L.; Ma, G.W. Characteristics of biodiversity spatial differentiation in the Chengdu-Chongqing economic zone. Res. Environ. Sci. 2012, 25, 1148–1154. [Google Scholar]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, G.D.; Duan, Y.F.; Fan, Y.; Zhao, Y.P.; Cheng, J.R.; Wang, X.R. Study on major pollinators and their flower-visiting behavior of Osmanthus fragrans. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2014, s1, 47–50. [Google Scholar]
- Luo, C.W.; Li, K.; Chen, X.M.; Chen, Y.; Sun, Y.Y. Foraging and main pollinators of Jatropha curcas in dry-hot valley. Chin. Bull. Entomol. 2008, 45, 121–127. [Google Scholar]
- Shen, J.S.; Qi, H.P.; Guo, Y.; Shao, Y.Q.; Liu, Y.M. Investigation on Sunflower Pollinating Insects. Apic. China 2008, 59, 27–28. [Google Scholar]
- Ebeling, A.; Klein, A.M.; Schumacher, J.; Weisser, W.W.; Tscharntke, T. How does plant richness affect pollinator richness and temporal stability of flower visits? Oikos 2010, 117, 1808–1815. [Google Scholar] [CrossRef]
- Elisabeth, J.E.; Sarah, C.K.; Greenleaf, S.; Garber, A.K.; Klein, A.M. Contribution of pollinator-mediated crops to nutrients in the human food supply. PLoS ONE 2011, 6, e21363. [Google Scholar]
- Hudewenz, A.; Pufal, G.; Bogeholz, A.L.; Klein, A.M. Cross-pollination benefits differ among oilseed rape varieties. J. Agric. Sci. 2014, 152, 770–778. [Google Scholar] [CrossRef]
- Huang, C.D.; Zhang, J.; Yang, W.J.; Tang, X. Spatiotemporal variation of carbon storage in forest vegetation in Sichuan Province. Chin. J. Appl. Ecol. 2007, 18, 2687–2692. [Google Scholar]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Mandelik, Y.; Winfree, R.; Neeson, T.; Kremen, C. Complementary habitat use by wild bees in agro-natural landscapes. Ecol. Appl. 2012, 22, 1535. [Google Scholar] [CrossRef]
- Heard, M.S.; Carvell, C.; Carreck, N.L.; Rothery, P.; Osborne, J.L.; Bourke, A.F.G. Landscape context not patch size determines bumble-bee density on flower mixtures sown for agri-environment schemes. Boil. Lett. 2007, 3, 638–641. [Google Scholar] [CrossRef] [Green Version]
- Williams, N.M.; Kremen, C. Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol. Appl. 2007, 17, 910–921. [Google Scholar] [CrossRef]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. Biol. Sci. 2003, 270, 955–961. [Google Scholar] [CrossRef]
- Winfree, R.; Griswold, T.; Kremen, C. Effect of human disturbance on bee communities in a forested ecosystem. Conserv. Biol. 2007, 21, 213–223. [Google Scholar] [CrossRef]
- Jha, S.; Vandermeer, J.H. Impacts of coffee agroforestry management on tropical bee communities. Boil. Conserv. 2010, 143, 1423–1431. [Google Scholar] [CrossRef]
- Holzschuh, A.; Steffandewenter, I.; Tscharntke, T. How do landscape composition and configuration, organic farming and fallow strips affect the diversity of bees, wasps and their parasitoids? J. Anim. Ecol. 2010, 79, 491–500. [Google Scholar] [CrossRef]
- Ramosjiliberto, R.; Albornoz, A.A.; Valdovinos, F.S.; Ramírez, C.S.; Arim, M.; Armesto, J.J.; Marquet, K.A. A network analysis of plant-pollinator interactions in temperate rain forests of Chiloe Island, Chile. Oecologia 2009, 160, 697–706. [Google Scholar] [CrossRef]
- Ferreira, P.A.; Boscolo, D.; Viana, B.F. What do we know about the effects of landscape changes on plant–pollinator interaction networks? Ecol. Indic. 2013, 31, 35–40. [Google Scholar] [CrossRef]
- Taki, H.; Kevan, P.G.; Ascher, J.S. Landscape effects of forest loss in a pollination system. Landsc. Ecol. 2007, 22, 1575–1587. [Google Scholar] [CrossRef] [Green Version]
- Priess, J.A.; Mimler, M.; Klein, A.M.; Schwarntke, T.; Dewenter, I.S. Linking deforestation scenarios to pollination services and ecoonomic returns in coffee agroforestry systems. Ecol. Appl. 2007, 17, 407–417. [Google Scholar] [CrossRef]
- Breeze, T.D.; Bailey, A.P.; Balcombe, K.G.; Potts, S.G. Pollination services in the UK: How important are honeybees? Agric. Ecosyst. Environ. 2011, 142, 137–143. [Google Scholar] [CrossRef]
- Williams, N.M.; Crone, E.E.; Roulston, T.H.; Minckley, R.L.; Packer, L.; Potts, S.G. Ecological and life-history traits predict bee species responses to environmental disturbances. Boil. Conserv. 2010, 143, 2280–2291. [Google Scholar] [CrossRef]
- Olschewski, R.; Tscharntke, T.; Benitez, P.C.; Schwarze, S.; Klein, A.M. Economic Evaluation of Pollination Services Comparing Coffee Landscapes in Ecuador and Indonesia. Ecol. Soc. 2006, 11, 709–723. [Google Scholar] [CrossRef]
- Cane, J.H.; Minckley, R.L.; Kervin, L.J.; Roulston, T.H.; Williams, N.M. Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol. Appl. 2006, 16, 632–644. [Google Scholar] [CrossRef]
- Leong, M.; Kremen, C.; Roderick, G.K. Pollinator interactions with yellow starthistle (Centaurea solstitialis) across urban, agricultural, and natural landscapes. PLoS ONE 2013, 9, e86357. [Google Scholar] [CrossRef]
- Benjamin, F.E.; Reilly, J.R.; Winfree, R. Pollinator body size mediates the scale at which land use drives crop pollination services. J. Appl. Ecol. 2014, 51, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Erik, Ö.; Henrikg, S. Semi-natural grasslands as population sources for pollinating insects in agricultural landscapes. J. Appl. Ecol. 2007, 44, 50–59. [Google Scholar]
- Carvalheiro, L.G.; Seymour, C.L.; Veldtman, R.; Nicolson, S.W. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J. Appl. Ecol. 2010, 47, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Blüthgen, N.; Klein, A.M. Functional complementarity and specialisation: The role of biodiversity in plant–pollinator interactions. Basic Appl. Ecol. 2011, 12, 282–291. [Google Scholar] [CrossRef]
- Williams, N.M.; Minckley, R.L.; Silverira, F.A. Variation in native bee faunas and its implications for detecting community changes. Ecol. Soc. 2001, 5, 803–805. [Google Scholar] [CrossRef]
- Kenta, T.; Inari, N.; Nagamitsu, T.; Goka, K.; Hiura, T. Commercialized European bumblebee can cause pollination disturbance: An experiment on seven native plant species in Japan. Boil. Conserv. 2007, 134, 298–309. [Google Scholar] [CrossRef]
- Greenleaf, S.S.; Williams, N.M.; Winfree, R.; Kremen, C. Bee Foraging Ranges and Their Relationship to Body Size. Oecologia 2007, 153, 589–596. [Google Scholar] [CrossRef]
- Brittain, C.; Williams, N.; Kremen, C.; Klein, A.M. Synergistic effects of non-Apis bees and honey bees for pollination services. Proc. Biol. Sci. 2013, 280, 20122767. [Google Scholar] [CrossRef]
- Albrecht, M.; Müller, C.B. Diverse pollinator communities enhance plant reproductive success. Proc. Biol. Sci. 2012, 279, 4845–4852. [Google Scholar] [CrossRef] [Green Version]
- Kremen, C.; Williams, N.M.; Bugg, R.L.; Fay, J.P.; Thorp, R.W. The area requirements of an ecosystem service: Crop pollination by native bee communities in California. Ecol. Lett. 2004, 7, 1109–1119. [Google Scholar] [CrossRef]
- Roulston, T.A.H.; Goodell, K. The Role of Resources and Risks in Regulating Wild Bee Populations. Annu. Rev. Entomol. 2011, 56, 293. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Matteson, K.C.; Xiao, L.; Silva, A.M.; Minor, E.S. Humans, bees, and pollination services in the city: The case of Chicago, IL (USA). Biodivers. Conserv. 2014, 23, 2857–2874. [Google Scholar] [CrossRef]
- Cresswell, J.E. A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 2011, 20, 149–157. [Google Scholar] [CrossRef]
- Brittain, C.; Potts, S.G. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011, 12, 321–331. [Google Scholar] [CrossRef]
- Di, P.G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [Green Version]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S.; Vanengelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Die Naturwiss. 2012, 99, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Wratten, S.D.; Gillespie, M.; Decourtye, A.; Mader, E.; Desneux, N. Pollinator habitat enhancement: Benefits to other ecosystem services. Agric. Ecosyst. Environ. 2012, 159, 112–122. [Google Scholar] [CrossRef]
- Melathopoulos, A.P.; Cutler, G.C.; Tyedmers, P. Where is the value in valuing pollination ecosystem services to agriculture? Ecol. Econ. 2015, 109, 59–70. [Google Scholar] [CrossRef]
- Brosi, B.J.; Daily, G.C.; Shih, T.M.; Oviedo, F.; Duran, G. The effects of forest fragmentation on bee communities in tropical countryside. J. Appl. Ecol. 2008, 45, 773–783. [Google Scholar] [CrossRef]
- Kohler, F.; Verhulst, J.; van Klink, R.; Kleijn, D. At What Spatial Scale Do High-Quality Habitats Enhance the Diversity of Forbs and Pollinators in Intensively Farmed Landscapes? J. Appl. Ecol. 2008, 45, 753–762. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2016, 12, 439–447. [Google Scholar] [CrossRef]
- Antonia, Z.; Lisa, L.; Jeannine, K.; Andreas, M.; Silke, H.; Silvia, D. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Boil. Conserv. 2010, 143, 669–676. [Google Scholar]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Xu, P.; Yan, K.; Guo, Y. Pollination Services from Insects in Homegardens in the Chengdu Plain will be Confronted with Crises. Sustainability 2019, 11, 2169. https://doi.org/10.3390/su11072169
Liu Q, Xu P, Yan K, Guo Y. Pollination Services from Insects in Homegardens in the Chengdu Plain will be Confronted with Crises. Sustainability. 2019; 11(7):2169. https://doi.org/10.3390/su11072169
Chicago/Turabian StyleLiu, Qin, Pei Xu, Kun Yan, and Yingman Guo. 2019. "Pollination Services from Insects in Homegardens in the Chengdu Plain will be Confronted with Crises" Sustainability 11, no. 7: 2169. https://doi.org/10.3390/su11072169