Arsenic, Oxidative Stress and Reproductive System
Abstract
:1. Introduction
2. Oxidative Stress
3. Role of Oxidative Stress via Genetics Causes in Infertility
4. Arsenic and Oxidative Stress
- -
- -
- The methylation of arsenic. The detoxification of arsenic is associated with its methylation in the liver by As3MT, and the production of its methylated metabolites include MMAV, MMAIII, DMAV, and DMAIII. In this pathway, arsenic needs glutathione (GSH) and other thiols. Depleting GSH and other thiols alters the redox status, producing arsenic methylated metabolites that increase oxidative stress [44,45,46].
- -
- The alteration of some signaling pathways: such as the tyrosine phosphorylation pathway and mitogen-activated protein kinase (MAPK) pathway, and transcription factors such as NF-kB, AP-1, apoptosis, the activation of p53, and Bax expression [47].
- -
- Damage to proteins, carbohydrates, lipids, and DNA. Arsenic causes damage to protein by producing •OH or O2•- that leads to the production of carbonyl, aldehydes, and keto compounds. This metalloid also damages some amino acid residues such as cysteine and methionine, and this may lead to alterations in protein structure, degradation, unfolding, fragmentation, the inactivation of enzymes (such as antioxidant enzymes, pyruvate dehydrogenase), and the production of advanced glycation end products (AGEs) [27].
5. Infertility and Oxidative Stress
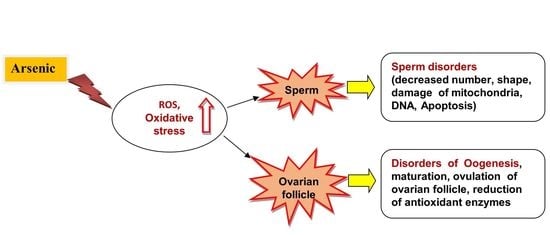
6. Arsenic Toxicity and Male and Female Reproductive Systems
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviation
References
- World Health Organization. WHO fact sheet on infertility. Glob. Reprod. Health 2021, 6, e52. Available online: https://journals.lww.com/grh/Fulltext/2021/01010/WHO_fact_sheet_on_infertility.1.aspx (accessed on 8 January 2021). [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Evidence-based treatments for couples with unexplained infertility: A guideline. Fertil. Steril. 2020, 113, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Xu, W.; Huang, Q.; Liu, L.; Tian, M.; Xia, Y.; Zhang, W.; Shen, H. Low-level environmental arsenic exposure correlates with unexplained male infertility risk. Sci. Total Environ. 2016, 571, 307–313. [Google Scholar] [CrossRef]
- Erkan, M.; Aydin, Y.; Yilmaz, B.O.; Yildizbayrak, N. Arsenic-induced oxidative stress in reproductive systems. Toxicology 2021, 1, 145–155. [Google Scholar]
- Rami, Y.; Ebrahimpour, K.; Maghami, M.; Shoshtari-Yeganeh, B.; Kelishadi, R. The Association Between Heavy Metals Exposure and Sex Hormones: A Systematic Review on Current Evidence. Biol. Trace Elem. Res. 2022, 200, 3491–3510. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Ye, X.; Zhu, Z.; Li, C.; Zhou, J.; Liu, J. A case-control study of arsenic exposure with the risk of primary ovarian insufficiency in women. Environ. Sci. Pollut. Res. 2020, 27, 25220–25229. [Google Scholar] [CrossRef]
- Lei, H.L.; Wei, H.J.; Ho, H.Y.; Liao, K.W.; Chien, L.C. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: A cross-sectional study from Taiwan. BMC Public Health 2015, 15, 1220. [Google Scholar] [CrossRef] [Green Version]
- Renu, K.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Vinayagam, S.; Gopalakrishnan, A.V. Review on molecular and biochemical insights of arsenic-mediated male reproductive toxicity. Life Sci. 2018, 212, 37–58. [Google Scholar] [CrossRef]
- Barsøe, I.M.; Ebdrup, N.H.; Clausen, H.S.; Lyngsø, J.; Schullehner, J.; Ramlau-Hansen, C.H.; Knudsen, U.B. Drinking Water Arsenic and Adverse Reproductive Outcomes in Men and Women: A Systematic PRISMA Review. Water 2021, 13, 1885. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, L.; Alamdar, A.; Zhang, J.; Liu, L.; Tian, M.; Eqani, S.A.; Shen, H. Integrated proteomics and metabolomics analysis of rat testis: Mechanism of arsenic-induced male reproductive toxicity. Sci. Rep. 2016, 6, 32518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wai, K.M.; Umezaki, M.; Mar, O.; Umemura, M.; Watanabe, C. Arsenic exposure through drinking Water and oxidative stress Status: A cross-sectional study in the Ayeyarwady region, Myanmar. J. Trace Elem. Med. Biol. 2019, 54, 103–109. [Google Scholar]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med. Cell Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The.effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Aitken, R.J. Impact of oxidative stress on male and female germ cells: Implications for fertility. Reproduction 2020, 159, R189–R201. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef]
- Hoque, S.M.; Umehara, T.; Kawai, T.; Shimada, M. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radic. Biol. Med. 2021, 163, 344–355. [Google Scholar] [CrossRef]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.A.; Zarkovic, N. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Latchoumycandane, C.; Vaithinathan, S.; D’Cruz, S.C.; Mathur, P.P. Apoptosis and male infertility. In Male Infertility; Springer: Cham, Switzerland, 2020; pp. 479–486. [Google Scholar]
- Dutta, S.; Sengupta, P. The Role of Nitric Oxide on Male and Female Reproduction. Malays. J. Med. Sci. MJMS 2022, 29, 18. [Google Scholar] [PubMed]
- Lorian, K.; Kadkhodaee, M.; Kianian, F.; Abdi, A.; Sadeghipour, H.; Seifi, B. Oxidative stress, nitric oxide and inflammation in the pathophysiology of varicocele and the effect of hydrogen sulfide as a potential treatment. J. Physiol. Pharmacol. 2019, 23, 249–260. [Google Scholar]
- Pujianto, D.A.; Oktarina, M.; Sharma Sharaswati, I.A.; Yulhasri. Hydrogen peroxide has adverse effects on human sperm quality parameters, induces apoptosis, and reduces survival. J. Hum. Reprod. Sci. 2021, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Peña, F.J.; O’Flaherty, C.; Ortiz Rodríguez, J.M.; Martín Cano, F.E.; Gaitskell-Phillips, G.L.; Gil, M.C.; Ortega Ferrusola, C. Redox regulation and oxidative stress: The particular case of the stallion spermatozoa. Antioxidants 2019, 8, 567. [Google Scholar] [CrossRef] [Green Version]
- Zargari, F. Arsenic and Oxidative Stress: An Overview. In Arsenic Toxicity: Challenges and Solutions, 1st ed.; Kumar, N., Ed.; Springer: Singapore, 2021; pp. 27–63. [Google Scholar] [CrossRef]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Gentile, F. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in aging-related disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Roy, B. Physiology of stress and the involvement of reactive oxidative species: A mini-review. Quest Int. J. Med. Health Sci. 2018, 1, 19–24. [Google Scholar]
- Berby, B.; Bichara, C.; Rives-Feraille, A.; Jumeau, F.; Pizio, P.D.; Sétif, V.; Sibert, L.; Dumont, L.; Rondanino, C.; Rives, N. Oxidative Stress Is Associated with Telomere Interaction Impairment and Chromatin Condensation Defects in Spermatozoa of Infertile Males. Antioxidants 2021, 10, 593. [Google Scholar] [CrossRef]
- Rocca, M.S.; Foresta, C.; Ferlin, A. Telomere length: Lights and shadows on their role in human reproduction. Biol. Reprod. 2019, 100, 305–317. [Google Scholar] [CrossRef]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. The Role of Genetics and Oxidative Stress in the Etiology of Male Infertility—A Unifying Hypothesis? Front. Endocrinol. 2020, 11, 581838. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, J.; Lou, B.; Wu, R.; Wang, G.; Lu, C.; Wang, H.; Pi, J.; Xu, Y. The Role of Reactive Oxygen Species in Arsenic Toxicity. Biomolecules 2020, 10, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Park, N.Y.; Kang, K.; Calderwood, S.K.; Cho, D.H.; Bae, I.J.; Bunch, H. Arsenic hexoxide has differential effects on cell proliferation and genome-wide gene expression in human primary mammary epithelial and MCF7 cells. Sci. Rep. 2021, 11, 3761. [Google Scholar] [CrossRef] [PubMed]
- Taşçı, T.; Eldem, V.; Erkan, M. Sodium Arsenic Alters the Gene Expression of some Steroidogenic Genes in TM3 Leydig Cell. Celal Bayar Univ. J. Sci. 2019, 15, 265–270. [Google Scholar] [CrossRef]
- Eckstein, M.; Eleazer, R.; Rea, M.; Fondufe-Mittendorf, Y. Epigenomic reprogramming in inorganic arsenic-mediated gene expression patterns during carcinogenesis. Rev. Environ. Health 2017, 32, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmac. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Minatel, B.C.; Sage, A.P.; Anderson, C.; Hubaux, R.; Marshall, E.A.; Lam, W.L.; Martinez, V.D. Environmental arsenic exposure: From genetic susceptibility to pathogenesis. Environ. Int. 2018, 112, 183–197. [Google Scholar] [CrossRef]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [Green Version]
- Mishra, D.; Mehta, A.; Flora, S.J. Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: Role of glutathione and linked enzymes. Chem. Res. Toxicol. 2008, 21, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Németi, B.; Gregus, Z. Reduction of arsenate to arsenite in hepatic cytosol. Toxicol. Sci. 2002, 70, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dopp, E.; Kligerman, A.D.; Diaz-Bone, R.A. Organoarsenicals. Uptake, metabolism, and toxicity. Met. Ions Life Sci. 2010, 7, 231–265. [Google Scholar] [PubMed]
- Thomas, D.J.; Li, J.; Waters, S.B.; Xing, W.; Adair, B.M.; Drobna, Z.; Devesa, V.; Styblo, M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp. Biol. Med. 2007, 232, 3–13. [Google Scholar]
- Nagesh, R.; Kiran Kumar, K.M.; Naveen Kumar, M.; Patil, R.H.; Sharma, S.C. Stress activated p38 MAPK regulates cell cycle via AP-1 factors in areca extract exposed human lung epithelial cells. Cytotechnology 2019, 71, 507–520. [Google Scholar] [CrossRef]
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef]
- Wirtitsch, M.; Roth, E.; Bachleitner-Hofmann, T.; Wessner, B.; Sturlan, S. Omega-3 and omega-6 polyunsaturated fatty acids enhance arsenic trioxide efficacy in arsenic trioxide-resistant leukemic and solid tumor cells. Oncol. Res. 2009, 18, 83–94. [Google Scholar] [CrossRef]
- De Vizcaya-Ruiz, A.; Barbier, O.; Ruiz-Ramos, R.; Cebrian, M.E. Biomarkers of oxidative stress and damage in human populations exposed to arsenic. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 674, 85–92. [Google Scholar] [CrossRef]
- Scarlata, E.; O’Flaherty, C. Antioxidant enzymes and male fertility: Lessons from knockout models. Antioxid. Redox. Signal. 2020, 32, 569–580. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect. Prev. 2004, 28, 385–391. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Sengupta, P. Oxidative stress in pathologies of male reproductive disorders. In Pathology; Academic Press: Cambridge, MA, USA, 2020; pp. 15–27. [Google Scholar]
- Durairajanayagam, D. Physiological role of reactive oxygen species in male reproduction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Academic Press: Cambridge, MA, USA, 2019; pp. 65–78. [Google Scholar]
- Garrido, N.; Meseguer, M.; Simon, C.; Pellicer, A.; Remohi, J. Pro-oxidative and anti-oxidative imbalance in human semen and its relation with male fertility. Asian J. Androl. 2004, 6, 59–66. [Google Scholar] [PubMed]
- Thomas, J.; Fishel, S.B.; Hall, J.A.; Green, S.; Newton, T.A.; Thornton, S.J. Increased polymorphonuclear granulocytes in seminal plasma in relation to sperm morphology. Hum. Reprod. 1997, 12, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, D.; Moayeri, A.; Rostamzadeh, O.; Rostamzadeh, A.; Kebria, M.M. Reactive oxygenated species (ROS) in male fertility; source, interaction mechanism and antioxidant therapy. Res. J. Pharm. Technol. 2018, 11, 791–796. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Reactive oxygen species in seminal plasma as a cause of male infertility. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 565–572. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J., Jr.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Nsonwu-Anyanwu, A.C.; Ekong, E.R.; Offor, S.J.; Awusha, O.F.; Orji, O.C.; Umoh, E.I.; Usoro, C.A.O. Heavy metals, biomarkers of oxidative stress and changes in sperm function: A case-control study. Int. J. Reprod. Biomed. 2019, 17, 163. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Li, W.; Fang, Z.; Li, N.; Wu, S.; Hong, M. p53-and ROS-mediated AIF pathway involved in TGEV-induced apoptosis. J. Vet. Med. Sci. 2018, 80, 1775–1781. [Google Scholar] [CrossRef] [Green Version]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Terao, H.; Wada-Hiraike, O.; Nagumo, A.; Kunitomi, C.; Azhary, J.M.; Harada, M.; Osuga, Y. Role of oxidative stress in follicular fluid on embryos of patients undergoing assisted reproductive technology treatment. J. Obstet. Gynaecol. 2019, 45, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Chano, T. Linking oxidative stress and ovarian cancers. In Cancer; Academic Press: Cambridge, MA, USA; pp. 77–86.
- Mohammadi, M. Oxidative stress and polycystic ovary syndrome: A brief review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Hundal, S.S. Effect of sodium arsenite on reproductive organs of female Wistar rats. Arch. Environ. Occup. Health 2016, 71, 16–25. [Google Scholar] [CrossRef]
- Wirth, J.J.; Mijal, R.S. Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med. 2010, 56, 147–167. [Google Scholar] [CrossRef] [Green Version]
- Kippler, M.; Wagatsuma, Y.; Rahman, A.; Nermell, B.; Persson, L.Å.; Raqib, R.; Vahter, M. Environmental exposure to arsenic and cadmium during pregnancy and fetal size: A longitudinal study in rural Bangladesh. Reprod. Toxicol. 2012, 34, 504–511. [Google Scholar] [CrossRef]
- De Palma, G.; Ortiz, A.; Apostoli, P. Effects of metallic elements on reproduction and development. In Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2022; pp. 565–592. [Google Scholar]
- Jana, K.; Jana, S.; Samanta, P.K. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: Possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 2006, 4, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im Chang, S.; Jin, B.; Youn, P.; Park, C.; Park, J.D.; Ryu, D.Y. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol. Appl. Pharmacol. 2007, 218, 196–203. [Google Scholar] [CrossRef]
- Saberi Sis, F.; Zargari, F. The effect of aqueous extract of white tea on serum levels of FSH, LH and testosterone in rats exposed to arsenic. J. Fasa Univ. Med. Sci. 2017, 7, 398–405. [Google Scholar]
- Rosenblatt, A.E.; Burnstein, K.L. Inhibition of androgen receptor transcriptional activity as a novel mechanism of action of arsenic. J. Mol. Endocrinol. 2009, 23, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Kaltreider, R.C.; Davis, A.M.; Lariviere, J.P.; Hamilton, J.W. Arsenic alters the function of the glucocorticoid receptor as a transcription factor. Environ. Health Perspect. 2001, 109, 245–251. [Google Scholar] [CrossRef]
- Ilieva, I.; Sainova, I.; Yosifcheva, K. Toxic Effects of Heavy Metals (Mercury and Arsenic) on the Male Fertility. Acta Morphol. Anthropol. 2021, 28, 1–2. [Google Scholar]
- Shao, Y.; Zhao, H.; Wang, Y.; Liu, J.; Li, J.; Chai, H.; Xing, M. Arsenic and/or copper caused inflammatory response via activation of inducible nitric oxide synthase pathway and triggered heat shock protein responses in testis tissues of chicken. Environ. Sci. Pollut. Res. Int. 2018, 25, 7719–7729. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Ghosh, S.; Chaki, S.; Debnath, J.; Ghosh, D. Effect of sodium arsenite on plasma levels of gonadotrophins and ovarian steroidogenesis in mature albino rats: Duration-dependent response. J. Toxicol. Sci. 1999, 24, 425–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.B.M.; Reddy, P.S.; Sasikala, P.; Reddy, Y.V.K. Transplacental and lactational exposure of arsenic to mice: Effect on steroidogenic enzymes and hormones of male reproduction. Int. J. Toxicol. Pharmacol. Res. 2010, 2, 95–98. [Google Scholar]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]

| Negative Effects of Arsenic on the Male and Female Reproductive Systems | References |
|---|---|
| Reduction in the number of sperm (due to reduction in GSH and increased MDA) | [73] |
| Increase in the levels of ROS in testes | [73] |
| Alteration in hormone secretion (reduction in testosterone, FSH, LH) | [72,74] |
| Disruption of spermatogenesis by inhibition of androgen receptor activity | [75] |
| Interaction with the cysteine residues in DNA-binding domain (DBD) of steroid receptors inhibits their activity | [76] |
| Reduction in testicular weight | [9,77] |
| Alteration of some enzymes such as lactate dehydrogenase (LDH), acid phosphatase (ACP), γ-glutamyl transpeptidase (GGT) | [9] |
| Reduction in sperm motility and viability | [9,77] |
| Decrease in the expression level of CYP11A1, CYP17A1 | [37] |
| Impaired sperm acrosome membrane protein 1 (SPACA1) and alteration in shape of sperm head | [9,77] |
| Decrease in VDAC3 and disturbance of fertilization process | [11] |
| Induction of inflammation in testes and increase in the production of inflammatory factors such as TNF-α, COX, NF-kB, caspase 3 | [78] |
| Alteration of some regulator enzymes in steroidogenesis such as 3β-hydroxysteroid dehydrogenase (3-βHSD), 17β-hydroxysteroid dehydrogenase (17βHSD) due to low levels of gonadotropin | [77,78,79,80] |
| Reduction in gonadotropin secretion due to alteration of the levels of some neurotransmitters (reduction in LH, FSH, estradiol) | [77,81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargari, F.; Rahaman, M.S.; KazemPour, R.; Hajirostamlou, M. Arsenic, Oxidative Stress and Reproductive System. J. Xenobiot. 2022, 12, 214-222. https://doi.org/10.3390/jox12030016
Zargari F, Rahaman MS, KazemPour R, Hajirostamlou M. Arsenic, Oxidative Stress and Reproductive System. Journal of Xenobiotics. 2022; 12(3):214-222. https://doi.org/10.3390/jox12030016
Chicago/Turabian StyleZargari, Felor, Md. Shiblur Rahaman, Robab KazemPour, and Mahbobeh Hajirostamlou. 2022. "Arsenic, Oxidative Stress and Reproductive System" Journal of Xenobiotics 12, no. 3: 214-222. https://doi.org/10.3390/jox12030016