An Interprofessional Approach to Aural Rehabilitation for Adults with Hearing Loss and Cognitive Concerns
Abstract
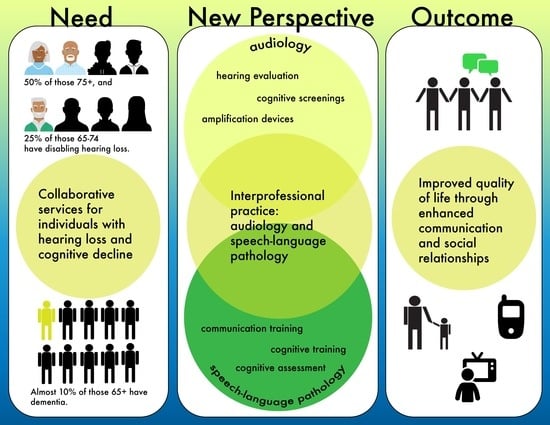
:1. Introduction
1.1. Continuum of Cognitive Decline
1.2. Relationship between Hearing Loss and Cognition
1.3. Hearing Loss and Comorbidities in the Presence of Cognitive Decline
1.4. Scope of Practice Related to Cognition
“Clinical service delivery areas include all aspects of hearing, balance, and other related disorders that impact hearing and balance, including areas of tinnitus, cognition, and auditory processing for individuals across the lifespan. ….Additional screening measures of mental health and cognitive impairment should be used to assess, treat, and refer”.
“Service delivery areas include all aspects of communication and swallowing and related areas that impact communication and swallowing: speech production, fluency, language, cognition, voice, resonance, feeding, swallowing, and hearing”.
2. New Perspective: An Interprofessional Approach to Aural Rehabilitation
2.1. Cognitive Screening
2.2. Hearing Technology
2.3. Instruction in Environmental Management, Communication Strategies, and Self Advocacy
2.4. Counseling: Supporting Emotional Well-Being of Clients and Caregivers
2.5. Lessons Learned
- Breaking complex information into smaller chunks;
- Sharing client-friendly printed information to support memory;
- Encouraging the practice of skills before the client leaves the clinic;
- Scheduling short breaks or multiple appointments to accommodate for attention and fatigue concerns and troubleshooting;
- Inviting caregivers to participate in appointments.
3. Conclusions
3.1. Relative Strengths of an Interprofessional Approach
3.2. Barriers to an Interprofessional Approach
3.3. Implications for Future Research and Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quick Statistics About Hearing|NIDCD. Available online: https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing (accessed on 19 September 2023).
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Wiley, T.L.; Nondahl, D.M. The Impact of Hearing Loss on Quality of Life in Older Adults. Gerontologist 2003, 43, 661–668. [Google Scholar] [CrossRef]
- Lin, F.R. Hearing Loss and Cognition among Older Adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1131–1136. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L.; Metter, E.J.; An, Y.; Zonderman, A.B.; Resnick, S.M. Hearing Loss and Cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology 2011, 25, 763–770. [Google Scholar] [CrossRef]
- Dupuis, K.; Pichora-Fuller, M.K.; Chasteen, A.L.; Marchuk, V.; Singh, G.; Smith, S.L. Effects of Hearing and Vision Impairments on the Montreal Cognitive Assessment. Aging Neuropsychol. Cogn. 2015, 22, 413–437. [Google Scholar] [CrossRef]
- Broome, E.E.; Tannirandorn, P.; Straus, J.; Beale, P.; Heffernan, E.; Dening, T.; Henshaw, H. Patient Perceptions of Cognitive Screening in Adult Audiology Services: A Qualitative Exploration. Front. Neurol. 2023, 14, 1143128. [Google Scholar] [CrossRef]
- Shen, J.; Anderson, M.C.; Arehart, K.H.; Souza, P.E. Using Cognitive Screening Tests in Audiology. Am. J. Audiol. 2016, 25, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Trajectories of Normal Cognitive Aging. Psychol. Aging 2019, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Reisberg, B.; Zaudig, M.; Petersen, R.C.; Ritchie, K.; Broich, K.; Belleville, S.; Brodaty, H.; Bennett, D.; Chertkow, H.; et al. Mild Cognitive Impairment. Lancet 2006, 367, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice Guideline Update Summary: Mild Cognitive Impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Csukly, G.; Sirály, E.; Fodor, Z.; Horváth, A.; Salacz, P.; Hidasi, Z.; Csibri, É.; Rudas, G.; Szabó, Á. The Differentiation of Amnestic Type MCI from the Non-Amnestic Types by Structural MRI. Front. Aging Neurosci. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Cognitive Impairment in Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020, 323, 757–763. [Google Scholar] [CrossRef]
- Understanding Different Types of Dementia. National Institute on Aging. Available online: https://www.nia.nih.gov/health/infographics/understanding-different-types-dementia (accessed on 22 September 2023).
- Muangpaisan, W.; Petcharat, C.; Srinonprasert, V. Prevalence of Potentially Reversible Conditions in Dementia and Mild Cognitive Impairment in a Geriatric Clinic. Geriatr. Gerontol. Int. 2012, 12, 59–64. [Google Scholar] [CrossRef]
- Przybelski, A.G.; Vogt, N.M.; Bendlin, B.B.; Przybelski, R.J. Vitamin Deficiencies in Geriatric Memory Patients. Alzheimers Dement. 2020, 16, e038765. [Google Scholar] [CrossRef]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022, 79, 1242–1249. [Google Scholar] [CrossRef]
- Surprenant, A.M.; DiDonato, R. Community-Dwelling Older Adults with Hearing Loss Experience Greater Decline in Cognitive Function over Time than Those with Normal Hearing. Evid. Based Nurs. 2014, 17, 60–61. [Google Scholar] [CrossRef]
- Huang, A.R.; Jiang, K.; Lin, F.R.; Deal, J.A.; Reed, N.S. Hearing Loss and Dementia Prevalence in Older Adults in the US. JAMA 2023, 329, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Pichora-Fuller, K. Is Hearing Loss in Older Adults Predictive of Later Development of Dementia and Does Hearing Care Modify Dementia Risk? Can. Audiol. 2023, 10, 1–26. [Google Scholar]
- Campbell, J.; Sharma, A. Cross-Modal Re-Organization in Adults with Early Stage Hearing Loss. PLoS ONE 2014, 9, e90594. [Google Scholar] [CrossRef]
- Lin, F.R.; Ferrucci, L.; An, Y.; Goh, J.O.; Doshi, J.; Metter, E.J.; Davatzikos, C.; Kraut, M.A.; Resnick, S.M. Association of Hearing Impairment with Brain Volume Changes in Older Adults. NeuroImage 2014, 90, 84–92. [Google Scholar] [CrossRef]
- Loughrey, D.G.; Kelly, M.E.; Kelley, G.A.; Brennan, S.; Lawlor, B.A. Association of Age-Related Hearing Loss With Cognitive Function, Cognitive Impairment, and Dementia: A Systematic Review and Meta-Analysis. JAMA Otolaryngol.-Head Neck Surg. 2018, 144, 115–126. [Google Scholar] [CrossRef]
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.-L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M.; et al. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 2013, 173, 293–299. [Google Scholar] [CrossRef]
- Weinstein, B.E.; Ventry, I.M. Hearing Impairment and Social Isolation in the Elderly. J. Speech Lang. Hear. Res. 1982, 25, 593–599. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Amieva, H.; Ouvrard, C.; Giulioli, C.; Meillon, C.; Rullier, L.; Dartigues, J.-F. Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J. Am. Geriatr. Soc. 2015, 63, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Pike, J.R.; Albert, M.S.; Arnold, M.; Burgard, S.; Chisolm, T.; Couper, D.; Deal, J.A.; Goman, A.M.; Glynn, N.W.; et al. Hearing Intervention versus Health Education Control to Reduce Cognitive Decline in Older Adults with Hearing Loss in the USA (ACHIEVE): A Multicentre, Randomised Controlled Trial. Lancet 2023, 402, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.S.; Clifton, L.; Kuźma, E.; Littlejohns, T.J. Speech-in-Noise Hearing Impairment Is Associated with an Increased Risk of Incident Dementia in 82,039 UK Biobank Participants. Alzheimers Dement. J. Alzheimers Assoc. 2022, 18, 445–456. [Google Scholar] [CrossRef]
- Gates, G.A.; Cobb, J.L.; Linn, R.T.; Rees, T.; Wolf, P.A.; D’Agostino, R.B. Central Auditory Dysfunction, Cognitive Dysfunction, and Dementia in Older People. Arch. Otolaryngol. Neck Surg. 1996, 122, 161–167. [Google Scholar] [CrossRef]
- Gates, G.A.; Anderson, M.L.; McCurry, S.M.; Feeney, M.P.; Larson, E.B. Central Auditory Dysfunction as a Harbinger of Alzheimer Dementia. Arch. Otolaryngol. Neck Surg. 2011, 137, 390–395. [Google Scholar] [CrossRef]
- Ehrlich, J.R.; Goldstein, J.; Swenor, B.K.; Whitson, H.; Langa, K.M.; Veliz, P. Addition of Vision Impairment to a Life-Course Model of Potentially Modifiable Dementia Risk Factors in the US. JAMA Neurol. 2022, 79, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Deal, J.; Rojas, J.C. Visual Impairment as a Modifiable Risk Factor in Dementia Prevention and Management. JAMA Neurol. 2022, 79, 542–543. [Google Scholar] [CrossRef]
- Carment, L.; Abdellatif, A.; Lafuente-Lafuente, C.; Pariel, S.; Maier, M.A.; Belmin, J.; Lindberg, P.G. Manual Dexterity and Aging: A Pilot Study Disentangling Sensorimotor from Cognitive Decline. Front. Neurol. 2018, 9, 910. [Google Scholar] [CrossRef]
- Abe, T.; Soma, Y.; Kitano, N.; Jindo, T.; Sato, A.; Tsunoda, K.; Tsuji, T.; Okura, T. Change in Hand Dexterity and Habitual Gait Speed Reflects Cognitive Decline over Time in Healthy Older Adults: A Longitudinal Study. J. Phys. Ther. Sci. 2017, 29, 1737–1741. [Google Scholar] [CrossRef]
- Kluger, A.; Gianutsos, J.G.; Golomb, J.; Ferris, S.H.; George, A.E.; Franssen, E.; Reisberg, B. Patterns of Motor Impairement in Normal Aging, Mild Cognitive Decline, and Early Alzheimer’s Disease. J. Gerontol. B Psychol. Sci. Soc. Sci. 1997, 52B, P28–P39. [Google Scholar] [CrossRef]
- Scope of Practice in Audiology. American Speech-Language-Hearing Association. Available online: https://www.asha.org/policy/sp2018-00353/ (accessed on 5 September 2023).
- Black, S.; Souza, P.E. Cognitive-Screening Practices among Audiologists. Audiol. Today 2020, 32, 46–53. [Google Scholar]
- Scope of Practice in Speech-Language Pathology. American Speech-Language-Hearing Association. Available online: https://www.asha.org/policy/sp2016-00343/ (accessed on 5 September 2023).
- Boothroyd, A. Adult Aural Rehabilitation: What Is It and Does It Work? Trends Amplif. 2007, 11, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tye-Murray, N. Foundations of Aural Rehabilitation: Children, Adults, and Their Family Members; Plural: San Diego, CA, USA, 2022. [Google Scholar]
- Beck, D.L.; Grisel, J.J. Cognitive Screenings in Otolaryngology? The Time Has Come. J. Otolaryngol.-ENT Res. 2022, 14, 56–60. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Tumosa, N. Saint Louis University Mental Status Examination; American Psychological Association: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Cahn-Hidalgo, D.; Estes, P.W.; Benabou, R. Validity, Reliability, and Psychometric Properties of a Computerized, Cognitive Assessment Test (Cognivue®). World J. Psychiatry 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Marinelli, J.P.; Lohse, C.M.; Fussell, W.L.; Petersen, R.C.; Reed, N.S.; Machulda, M.M.; Vassilaki, M.; Carlson, M.L. Association between Hearing Loss and Development of Dementia Using Formal Behavioural Audiometric Testing within the Mayo Clinic Study of Aging (MCSA): A Prospective Population-Based Study. Lancet Healthy Longev. 2022, 3, e817–e824. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, L.; Azzarello, J.; Baldwin, J.; Ciro, C.; Hudson, M.A.; Johnson, C.E.; John, A.B. The Impact of Amplification on Cognitive Screening Test Scores. J. Gerontol. Nurs. 2022, 48, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Uchanski, R. Clear Speech. In The Handbook of Speech Perception; John Wiley and Sons: Hoboken, NJ, USA, 2008; pp. 207–235. [Google Scholar]
- Foo, C.; Rudner, M.; Rönnberg, J.; Lunner, T. Recognition of Speech in Noise with New Hearing Instrument Compression Release Settings Requires Explicit Cognitive Storage and Processing Capacity. J. Am. Acad. Audiol. 2007, 18, 618–631. [Google Scholar] [CrossRef]
- Lunner, T.; Sundewall-Thorén, E. Interactions between Cognition, Compression, and Listening Conditions: Effects on Speech-in-Noise Performance in a Two-Channel Hearing Aid. J. Am. Acad. Audiol. 2007, 18, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.V.; Mulla, R.; Dervin, E.; Coyan, K.C. HearCARE: Hearing and Communication Assistance for Resident Engagement. Semin. Hear. 2017, 38, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Margolis, R.H. What Do Your Patients Remember? Hear. J. 2004, 57, 10. [Google Scholar] [CrossRef]
- Weiss, B.D. Health Literacy and Patient Safety: Help Patients Understand. Manual for Clinicians, 2nd ed.; American Medical Association Foundation: Chicago, IL, USA, 2010. [Google Scholar]
- Westby, C.; Burda, A.; Mehta, Z. Asking the Right Questions in the Right Ways. ASHA Lead. 2003, 8, 4–17. [Google Scholar] [CrossRef]
- Mamo, S.K.; Nirmalasari, O.; Nieman, C.L.; McNabney, M.K.; Simpson, A.; Oh, E.S.; Lin, F.R. Hearing Care Intervention for Persons with Dementia: A Pilot Study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Payne, J.C. Supporting Family Caregivers of Adults with Communication Disorders: A Resource Guide for Speech-Language Pathologists and Audiologists; Plural: San Diego, CA, USA, 2015. [Google Scholar]
- Cultural Responsiveness. Available online: https://www.asha.org/practice-portal/professional-issues/cultural-responsiveness/ (accessed on 3 January 2024).
- Gildersleeve-Neumann, C. Cultural Considerations for Dementia. Available online: https://sites.google.com/pdx.edu/multicsd/home (accessed on 5 January 2024).
- National Standards for Culturally and Linguistically Appropriate Services (CLAS) in Health and Health Care. Available online: https://thinkculturalhealth.hhs.gov/assets/pdfs/EnhancedNationalCLASStandards.pdf (accessed on 5 January 2024).
- How to Use the ICF—A Practical Manual for Using the International Classification of Functioning, Disability and Health. Available online: https://www.who.int/publications/m/item/how-to-use-the-icf---a-practical-manual-for-using-the-international-classification-of-functioning-disability-and-health (accessed on 5 January 2024).
- Luterman, D. Counseling Persons with Communication Disorders and Their Families; Pro-Ed: Austin, TX, USA, 2017. [Google Scholar]
- McFarlane, L.-A. Motivational Interviewing: Practical Strategies for Speech-Language Pathologists and Audiologists. Can. J. Speech-Lang. Pathol. Audiol. 2012, 36, 8–17. [Google Scholar]
- Pichora-Fuller, K. Auditory and Cognitive Processing in Audiologic Rehabilitation. In Adult Audiologic Rehabilitation; Plural: San Diego, CA, USA, 2021; pp. 551–572. [Google Scholar]
- COSI—NAL. Available online: https://www.nal.gov.au/nal_products/cosi/ (accessed on 22 September 2023).
- Thibodeau, L.M. Plotting beyond the Audiogram to the TELEGRAM, a New Assessment Tool. Hear. J. 2004, 57, 46–51. [Google Scholar] [CrossRef]
- Davis, T.; Choi, D.; Gordon-Hickey, S.; Estis, J. Interprofessional Collaborative Practice Trends Between Speech-Language Pathologists and Audiologists. J. Allied Health 2021, 50, 104–113. [Google Scholar]
- Davis, T.; Gordon-Hickey, S.; Choi, D.; Estis, J. Interprofessional Collaboration between Audiologists, SLPs. Hear. J. 2021, 74, 40–42. [Google Scholar] [CrossRef]
- Messersmith, J.; Brouwer, K. Student Perspectives of an Interdisciplinary Approach to Clinical Provision and Supervision. ASHA Perspect. Issues High. Educ. 2012, 15, 38–43. [Google Scholar] [CrossRef]
- Davis, T.; Choi, D.; Estis, J.; Gordon-Hickey, S. Understanding Neurogenic Communication Disorders in a Collaborative Context: A Team-Based Interprofessional Education Approach for Speech-Language Pathology and Audiology Students. Perspect. ASHA Spec. Interest Groups 2019, 4, 307–312. [Google Scholar] [CrossRef]
- O’Daniel, M.; Rosenstein, A.H. Professional Communication and Team Collaboration. In Patient Safety and Quality: An Evidence-Based Handbook for Nurses; Hughes, R.G., Ed.; Advances in Patient Safety; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Cagnin, A.; Jelcic, N.; Agostini, M.; Meneghello, F.; Parise, S.; Galano, A.; Tonin, P.; Dam, M.; Busse, C. Feasibility and efficacy of cognitive telerehabilitation in early Alzheimer’s disease: A pilot study. Clin. Interv. Aging 2014, 9, 1605–1611. [Google Scholar] [CrossRef]
- Weidner, K.; Lowman, J. Telepractice for Adult Speech-Language Pathology Services: A Systematic Review. Perspect. ASHA Spec. Interest Groups 2020, 5, 326–338. [Google Scholar] [CrossRef]
- Edgar, D.L.; Bargmann, P.L. Clinical Study of the Effectiveness of Constant Therapy in the Treatment of Clients With Dementia: Implications for Telepractice. Perspect. ASHA Spec. Interest Groups 2021, 6, 691–703. [Google Scholar] [CrossRef]
- Völter, C.; Schirmer, C.; Hinsen, D.; Roeber, M.; Dazert, S.; Bilda, K. Therapist-Guided Telerehabilitation for Adult Cochlear Implant Users: Developmental and Feasibility Study. JMIR Rehabil. Assist. Technol. 2020, 7, e15843. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, P.K.; Hughes, M.L. An Overview of Telepractice Applications for Comprehensive Cochlear Implant Service Delivery. Perspect. ASHA Spec. Interest Groups 2023, 8, 380–395. [Google Scholar] [CrossRef]
- Carter, J.M.; Killan, C.F.; Ridgwell, J.J. Telehealth Rehabilitation for Adults with Cochlear Implants in Response to the COVID-19 Pandemic: Platform Selection and Case Studies. Cochlear Implants Int. 2022, 23, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Preminger, J.; Nesbitt, L. Group Audiologic Rehabilitation for Adults: Justification and Implementation. In Adult Audiologic Rehabilitation; Plural: San Diego, CA, USA, 2014; pp. 307–328. [Google Scholar]
- Elman, R.J.; Bernstein-Ellis, E. The Efficacy of Group Communication Treatment in Adults With Chronic Aphasia. J. Speech Lang. Hear. Res. 1999, 42, 411–419. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tillery, K.H.; Rao, A. An Interprofessional Approach to Aural Rehabilitation for Adults with Hearing Loss and Cognitive Concerns. Audiol. Res. 2024, 14, 166-178. https://doi.org/10.3390/audiolres14010014
Tillery KH, Rao A. An Interprofessional Approach to Aural Rehabilitation for Adults with Hearing Loss and Cognitive Concerns. Audiology Research. 2024; 14(1):166-178. https://doi.org/10.3390/audiolres14010014
Chicago/Turabian StyleTillery, Kate Helms, and Aparna Rao. 2024. "An Interprofessional Approach to Aural Rehabilitation for Adults with Hearing Loss and Cognitive Concerns" Audiology Research 14, no. 1: 166-178. https://doi.org/10.3390/audiolres14010014