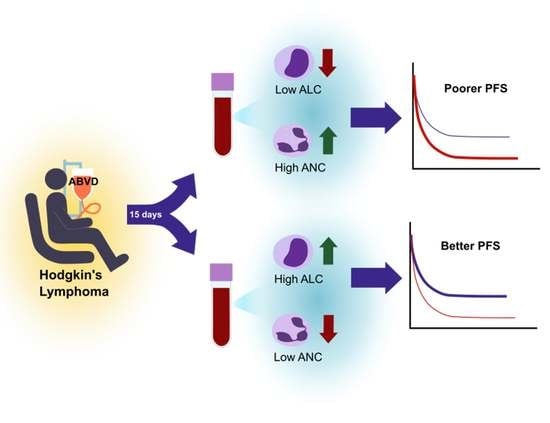
Post-Treatment Neutrophil and Lymphocyte Counts Predict Progression-Free Survival Following First-Line Chemotherapy in Hodgkin’s Lymphoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinicopathological Correlation
3.3. Survival Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hapgood, G.; Zheng, Y.; Sehn, L.H.; Villa, D.; Klasa, R.; Gerrie, A.S.; Shenkier, T.; Scott, D.W.; Gascoyne, R.D.; Slack, G.W.; et al. Evaluation of the Risk of Relapse in Classical Hodgkin Lymphoma at Event-Free Survival Time Points and Survival Comparison With the General Population in British Columbia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 2493–2500. [Google Scholar] [CrossRef]
- LaCasce, A.S. Treating Hodgkin lymphoma in the new millennium: Relapsed and refractory disease. Hematol. Oncol. 2019, 37 (Suppl. S1), 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kuppers, R. The biology of Hodgkin’s lymphoma. Nat. Rev. Cancer 2009, 9, 15–27. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Cézé, N.; Thibault, G.; Goujon, G.; Viguier, J.; Watier, H.; Dorval, E.; LeComte, T. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother. Pharmacol. 2011, 68, 1305–1313. [Google Scholar] [CrossRef]
- Song, M.K.; Chung, J.S.; Seol, Y.M.; Kim, S.G.; Shin, H.J.; Choi, Y.J.; Cho, G.J.; Shin, D.H. Influence of low absolute lymphocyte count of patients with nongerminal center type diffuse large B-cell lymphoma with R-CHOP therapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 140–144. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
- Miyamoto, R.; Inagawa, S.; Sano, N.; Tadano, S.; Adachi, S.; Yamamoto, M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2018, 44, 607–612. [Google Scholar] [CrossRef]
- Templeton, A.J.; Mcnamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Zhang, Z.; Chew, W.; Tan, G.F.; Lim, C.L.; Zhou, L.; Goh, W.L.; Poon, E.; Somasundaram, N.; Selvarajan, S.; et al. Biological significance and prognostic relevance of peripheral blood neutrophil-to-lymphocyte ratio in soft tissue sarcoma. Sci. Rep. 2018, 8, 11959. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.W.; Kang, H.J.; Park, C.; Yoon, D.H.; Kim, S.; Suh, C.; Kim, J.E.; Kim, C.-W.; Huh, J. Prognostic significance of the ratio of absolute neutrophil count to absolute lymphocyte count in classic Hodgkin lymphoma. Am. J. Clin. Pathol. 2012, 138, 846–854. [Google Scholar] [CrossRef]
- Ma, R.M.; Chen, C.Z.; Zhang, W.; You, J.; Huang, D.P.; Guo, G.L. Prognostic Value of Chemotherapy-Induced Neutropenia at the First Cycle in Invasive Breast Cancer. Medicine 2016, 95, e3240. [Google Scholar] [CrossRef]
- He, Y.; Li, T.; Liu, J.; Ou, Q.; Zhou, J. Early onset neutropenia: A useful predictor of chemosensitivity and favorable prognosis in patients with serous ovarian cancer. BMC Cancer 2020, 20, 116. [Google Scholar] [CrossRef]
- Hasenclever, D.; Diehl, V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Davuluri, R.; Jiang, W.; Fang, P.; Xu, C.; Komaki, R.; Gomez, D.R.; Welsh, J.; Cox, J.D.; Crane, C.H.; Hsu, C.C.; et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, C.; Liu, A.; van Rossum, P.S.; Deng, W.; Liao, Z.; Koong, A.C.; Mohan, R.; Lin, S.H. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 133, 9–15. [Google Scholar] [CrossRef]
- Abravan, A.; Faivre-Finn, C.; Kennedy, J.; McWilliam, A.; van Herk, M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J. Thorac. Oncol. 2020, 15, 1624–1635. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.M.; Cozen, W.; Steidl, C.; Carbone, A.; Hoppe, R.T.; Flechtner, H.H.; Bartlett, N.L. Hodgkin lymphoma. Nat. Rev. Dis. Prim. 2020, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Gurney, H. How to calculate the dose of chemotherapy. Br. J. Cancer 2002, 86, 1297–1302. [Google Scholar] [CrossRef]
- Bertholee, D.; Maring, J.G.; van Kuilenburg, A.B. Genotypes Affecting the Pharmacokinetics of Anticancer Drugs. Clin. Pharmacokinet. 2017, 56, 317–337. [Google Scholar] [CrossRef]
- Kurihara, T.; Kogo, M.; Ishii, M.; Shimada, K.; Yoneyama, K.; Kitamura, K.; Shimizu, S.; Yoshida, H.; Kiuchi, Y. Chemotherapy-induced neutropenia as a prognostic factor in patients with unresectable pancreatic cancer. Cancer Chemother. Pharmacol. 2015, 76, 1217–1224. [Google Scholar] [CrossRef]
- Cuccaro, A.; Bellesi, S.; Galli, E.; Zangrilli, I.; Corrente, F.; Cupelli, E.; Fatone, F.; Maiolo, E.; Alma, E.; Viscovo, M.; et al. PD-L1 expression in peripheral blood granulocytes at diagnosis as prognostic factor in classical Hodgkin lymphoma. J. Leukoc. Biol. 2022, 112, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Eldershaw, S.; Verma, K.; Croft, W.; Rai, T.; Kinsella, F.A.; Stephens, C.; Chen, H.; Nunnick, J.; Zuo, J.; Malladi, R.; et al. Lymphopenia-induced lymphoproliferation drives activation of naive T cells and expansion of regulatory populations. iScience 2021, 24, 102164. [Google Scholar]
- Verma, R.; Foster, R.E.; Horgan, K.; Mounsey, K.; Nixon, H.; Smalle, N.; Hughes, T.A.; Carter, C.R. Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res. BCR 2016, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.T.H.; Gargett, T.; Brown, M.P.; Ebert, L.M. Effects of Chemotherapy Agents on Circulating Leukocyte Populations: Potential Implications for the Success of CAR-T Cell Therapies. Cancers 2021, 13, 2225. [Google Scholar] [CrossRef]
- Taguchi, A.; Furusawa, A.; Ito, K.; Nakajima, Y.; Shimizuguchi, T.; Hara, K.; Takao, M.; Kashiyama, T.; Kino, N.; Karasawa, K.; et al. Postradiotherapy persistent lymphopenia as a poor prognostic factor in patients with cervical cancer receiving radiotherapy: A single-center, retrospective study. Int. J. Clin. Oncol. 2020, 25, 955–962. [Google Scholar] [CrossRef]
- Damen, P.J.; Kroese, T.E.; van Hillegersberg, R.; Schuit, E.; Peters, M.; Verhoeff, J.J.; Lin, S.H.; van Rossum, P.S. The Influence of Severe Radiation-Induced Lymphopenia on Overall Survival in Solid Tumors: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 936–948. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, K.J.; Han, S.C.; Seong, J. Effect of Interleukin-7 on Radiation-Induced Lymphopenia and Its Antitumor Effects in a Mouse Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1559–1569. [Google Scholar] [CrossRef]
- Bosco, N.; Agenes, F.; Ceredig, R. Effects of increasing IL-7 availability on lymphocytes during and after lymphopenia-induced proliferation. J. Immunol. 2005, 175, 162–170. [Google Scholar] [CrossRef]
- De Gast, G.C.; Vyth-Dreese, F.A.; Nooijen, W.; van den Bogaard, C.J.; Sein, J.; Holtkamp, M.M.; Linthorst, G.A.; Baars, J.W.; Schornagel, J.H.; Rodenhuis, S. Reinfusion of autologous lymphocytes with granulocyte-macrophage colony-stimulating factor induces rapid recovery of CD4+ and CD8+ T cells after high-dose chemotherapy for metastatic breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 58–64. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Number (%) |
|---|---|
| Total | 131 (100%) |
| Age | |
| Median age (years) | 29 (Range: 15–81) |
| Sex | |
| Male | 69 (52.7%) |
| Female | 62 (47.3%) |
| Ethnicity | |
| Chinese | 85 (64.9%) |
| Malay | 20 (15.3%) |
| Indian/Pakistani/Sri Lankan | 17 (13.0%) |
| Other | 9 (6.9%) |
| ECOG performance status | Can be |
| 0 | 85 (64.9%) |
| ≥1 | 46 (35.1%) |
| Ann Arbor stage | |
| I | 24(18.3%) |
| II | 68(51.9%) |
| III | 20 (15.3%) |
| IV | 19 (14.5%) |
| GHSG stage | |
| Early (87) | |
| Favorable | 40 (30.5%) |
| Unfavorable | 47 (35.9%) |
| Advanced (44) | |
| Stage IIBE/IIBX | 5 (3.8%) |
| Stage III | 20 (15.3%) |
| Stage IV | 19 (14.5%) |
| Histological subtype | |
| Nodular sclerosis | 100 (76.3%) |
| Mixed cellularity | 21 (16.0%) |
| Lymphocyte rich | 6 (4.6%) |
| Classical (NOS) | 4 (3.1%) |
| Bulky disease | |
| Present | 46 (35.1%) |
| Absent | 85 (64.9%) |
| B symptoms | |
| Present | 24 (18.3%) |
| Absent | 107 (81.7%) |
| Raised LDH | |
| Present | 64 (48.9%) |
| Absent | 67 (51.1%) |
| Bone marrow | |
| Involved | 13 (9.9%) |
| Not involved | 118 (90.1%) |
| Characteristic | Progression-Free Survival | |
|---|---|---|
| HR (95% CI) | p | |
| Age at diagnosis | 1.0073 (0.9791–1.0363) | 0.6173 |
| Ethnicity (Chinese vs. non-Chinese) | 0.7537 (0.2811–2.0203) | 0.5740 |
| Sex (male vs. female) | 0.7019 (0.2779–1.7729) | 0.4540 |
| B symptoms (present vs. absent) | 2.3562 (0.6712–8.2713) | 0.1810 |
| Bulky disease (present vs. absent) | 1.5425 (0.5837–4.0764) | 0.3821 |
| ECOG performance status (0 vs. ≥ 1) | 0.5104 (0.1962–1.3279) | 0.1680 |
| GHSG stage (advanced vs. early) | 1.7639 (0.6552–4.7492) | 0.2614 |
| Histological subtype (mixed cellular vs. other) | 0.8811 (0.2414–3.2160) | 0.8480 |
| pALC (low vs. high) | 3.9471 (1.5580–9.9995) | 0.0038 |
| pANC (high vs. low) | 2.9870 (1.0559–8.4504) | 0.0392 |
| pNLR (high vs. low) | N/A 5-year PFS 84% vs. 100% | 0.0078 |
| Raised LDH (present vs. absent) | 1.7852 (0.7066–4.5105) | 0.2204 |
| Characteristic | Progression-Free Survival | |
|---|---|---|
| HR (95% CI) | p | |
| pANC (high vs. low) | 6.978 (0.927–52.356) | 0.0592 |
| pALC (low vs. high) | 6.983 (1.604–30.395) | 0.0096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, G.F.; Goh, S.; Chang, E.W.Y.; Tan, Y.H.; Chiang, J.; Yang, V.S.; Poon, E.Y.L.; Somasundaram, N.; Bin Harunal Rashid, M.F.; Tao, M.; et al. Post-Treatment Neutrophil and Lymphocyte Counts Predict Progression-Free Survival Following First-Line Chemotherapy in Hodgkin’s Lymphoma. Hematol. Rep. 2023, 15, 108-118. https://doi.org/10.3390/hematolrep15010012
Tan GF, Goh S, Chang EWY, Tan YH, Chiang J, Yang VS, Poon EYL, Somasundaram N, Bin Harunal Rashid MF, Tao M, et al. Post-Treatment Neutrophil and Lymphocyte Counts Predict Progression-Free Survival Following First-Line Chemotherapy in Hodgkin’s Lymphoma. Hematology Reports. 2023; 15(1):108-118. https://doi.org/10.3390/hematolrep15010012
Chicago/Turabian StyleTan, Grace Fangmin, Siting Goh, Esther Wei Yin Chang, Ya Hwee Tan, Jianbang Chiang, Valerie Shiwen Yang, Eileen Yi Ling Poon, Nagavalli Somasundaram, Mohamad Farid Bin Harunal Rashid, Miriam Tao, and et al. 2023. "Post-Treatment Neutrophil and Lymphocyte Counts Predict Progression-Free Survival Following First-Line Chemotherapy in Hodgkin’s Lymphoma" Hematology Reports 15, no. 1: 108-118. https://doi.org/10.3390/hematolrep15010012