Unusual Presentation of Hodgkin’s Lymphoma in Pregnancy: A Case Report and Systematic Review of Literature
Abstract
:1. Introduction
2. Case Presentation
3. Methods Used for the Systematic Review of Literature
3.1. Search Criteria
3.2. Study Selection and Data Extraction
3.3. Search Results
4. Results
Characteristics of the Included Studies
5. Treatment
6. Pregnancy Outcome
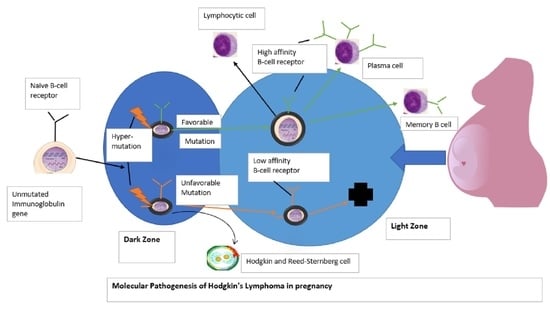
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Calsteren, K.; Heyns, L.; De Smet, F.; Van Eycken, L.; Gziri, M.M.; Van Gemert, W.; Halask, M.; Vergote, I.; Ottevanger, N.; Amant, F. Cancer During Pregnancy: An Analysis of 215 Patients Emphasizing the Obstetrical and the Neonatal Outcomes: 2010. J. Clin. Oncol. 2010, 28, 683–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amant, F.; Vandenbroucke, T.; Verheecke, M.; Fumagalli, M.; Halaska, M.J.; Boere, I.; Han, S.; Gziri, M.M.; Peccatori, F.; Rob, L.; et al. Pediatric Outcome after Maternal Cancer Diagnosed during Pregnancy. N. Engl. J. Med. 2015, 373, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Buchholtz, M.L.; Bucklein, V.; Brendel, M.; Paal, M. Superior vena cava syndrome related to mediastinal lymphoma in late pregnancy: A case report. Case Rep. Women Health 2018, 19, e00065. [Google Scholar] [CrossRef] [PubMed]
- Maggen, C.; Dierickx, D.; Lugtenburg, P.; Laenen, A.; Cardonick, E.; Smakov, R.G.; Bellido, M.; Cabrera-Garcia, A.; Gziri, M.M.; Halaska, M.J.; et al. Obstetrics and maternal outcomes in patients diagnosed with Hodgkin lymphoma during pregnancy: A multicenter, retrospective, cohort study. Lancet Haematol. 2019, 6, E551–E561. [Google Scholar] [CrossRef]
- De Sanctis, V.; Filippone, F.R.; Alfo, M.; Osti, M.F.; Minniti, G.; Enrici, R.M. Impact of Different Treatment Approaches on Pregnancy Outcome in Women Treated for Hodgkin Lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 755–761. [Google Scholar] [CrossRef]
- Aviles, A.; Nambo, M.J.; Neri, N. Treatment of Early Stages Hodgkin lymphoma during pregnancy. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018006. [Google Scholar] [CrossRef] [Green Version]
- Eyre, T.A.; Lau, J.; Mackillop, L.; Collins, G. Management, and controversies of classical Hodgkin lymphoma in pregnancy. Br. J. Haematol. 2015, 169, 613–630. [Google Scholar] [CrossRef]
- Gurevich-Shapiro, A.; Avivi, I. Current treatment of lymphoma in pregnancy. Expert. Rev. Hematol. 2019, 12, 449–459. [Google Scholar] [CrossRef]
- Moshe, Y.; Bentur, O.S.; Lishner, M.; Avivi, I. The management of Hodgkin lymphomas in pregnancies. Eur. J. Haematol. 2017, 99, 385–391. [Google Scholar] [CrossRef] [Green Version]
- El-Messidi, A.; Patenaude, V.; Hakeem, G.; Abenhaim, H.A. Incidence and outcomes of women with Hodgkin’s lymphoma in pregnancy: A population-based study on 7.9 million births. J. Perinat. Med. 2014, 43, 683–688. [Google Scholar] [CrossRef]
- Aisner, J.; Wiernik, P.H.; Pearl, P. Pregnancy outcome in patients treated for Hodgkin’s disease. J. Clin. Oncol. 1993, 11, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kortkontzelos, I.; Antoniou, N.; Akritidis, N.; Dernou, A.; Zagaliki, A.; Lykoudis, S. Hodgkin’s disease during pregnancy: A case report and review of the literature. Clin. Exp. Obstet. Gynecol. 2005, 32, 259–262. [Google Scholar]
- Dunleavy, K.; McLintock, C. How I treat lymphoma in pregnancy. Blood 2020, 136, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Bachanova, V.; Connors, J.M. Hodgkin Lymphoma in Pregnancy. Curr. Hematol. Malig. Rep. 2013, 8, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotteret, C.; Pham, Y.; Marcais, A.; Driessen, M.; Cisternini, S.; Schlatter, J. Maternal ABVD chemotherapy for Hodgkin lymphoma in a dichorionic diamniotic pregnancy: A case report. BMC Pregnancy Childbirth 2020, 20, 231. [Google Scholar] [CrossRef] [Green Version]
- Lishner, M.; Zemlickis, D.; Degendorfer, P.; Panzarella, T.; Sutcliffe, S.B.; Koren, G. Maternal and fetal outcome following Hodgkin’s disease in pregnancy. Cancer Pregnancy: Matern. Fetal Risks 1992, 65, 114–117. [Google Scholar]
- Anselmo, A.P.; Cavalieri, E.; Enrici, R.M.; Pescarmona, E.; Guerrisi, V.; Paesano, R.; Pachi, A.; Mandelli, F. Hodgkin’s disease during pregnancy: Diagnostic and therapeutic management. Fetal Diagn Ther. 1999, 14, 102–105. [Google Scholar] [CrossRef]
- Iriyama, N.; Horikoshi, A.; Tanaka, T.; Hirabayashi, Y.; Kodaira, H.; Hatta, Y.; Takeuchi, J. Successful treatment of Hodgkin lymphoma in second trimester of pregnancy: Feasibility of ABVD regimen. Int. J. Hematol. 2011, 94, 104–107. [Google Scholar] [CrossRef]
- Pileri, S.A.; Ascani, S.; Leoncini, L.; Sabattini, E.; Zinzani, P.L.; Piccaluga, P.P.; Pileri, A.; Giunti, M.; Falini, M.; Bolis, G.B.; et al. Hodgkin’s lymphoma: The pathologist’s viewpoint. J. Clin. Pathol. 2002, 55, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Brenner, B.; Avivi, I.; Lishner, M. Hematological cancers in pregnancy. Lancet 2012, 379, 580–587. [Google Scholar] [CrossRef]
- Ward, F.T.; Weiss, R.B. Lymphoma, and pregnancy. Semin. Oncol. 1989, 16, 397–409. [Google Scholar] [PubMed]
- Evens, A.M.; Advani, R.; Press, O.W.; Lossos, I.S.; Vose, J.M.; Hernandez-Ilizaliturri, F.J.; Robinson, B.K.; Otis, S.; Dagan, L.N.; Abdallah, R.; et al. Lymphoma occurring during pregnancy: Antenatal therapy, complications, and maternal survival in a multicenter analysis. J. Clin. Oncol. 2013, 31, 4132–4139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandenbriele, C.; Dierickx, D.; Amant, F.; Delforge, M. The treatment of hematologic malignancies in pregnancy. Facts Views Vis Obgyn. 2010, 2, 74–87. [Google Scholar]
- Bachanova, V.; Connors, J.M. How is Hodgkin lymphoma in pregnancy best treated? ASH Educ. Program Book 2008, 2008, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Gelb, A.B.; Van de Rijn, M.; Warnke, R.A.; Karnel, O.W. Pregnancy-associated lymphoma. A clinicopathologic study. Cancer 1996, 78, 304–310. [Google Scholar] [CrossRef]
- Thomas, P.R.; Bocheem, D.; Peckham, M.J. The Investigation and management of Hodgkin’s disease in the pregnant patient. Cancer 1976, 38, 1443–1451. [Google Scholar] [CrossRef]
- Avilés, A.; Díaz-Maqueo, J.C.; Talavera, A.; Guzmán, R.; García, E.L. Growth and development of children of mothers treated with chemotherapy during pregnancy: Current status of 43 children. Am. J. Hematol. 1991, 36, 243–248. [Google Scholar] [CrossRef]
- Dildy, G.A.; Moise, K.J.; Carpenter, R.J.; Klima, T. Maternal malignancy metastatic to the products of conception. A review. Obstet. Gynecol. Surv. 1989, 44, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Van Calsteren, K.; Halaska, M.J.; Gziri, M.M.; Hui, W.; Lagae, L.; Willemsen, M.A.; Kapusta, L.; Van Calster, B.; Wouters, H.; et al. Long-term cognitive and cardiac outcomes after prenatal exposure to chemotherapy in children aged 18 months or older: An observational study. Lancet Oncol. 2012, 13, 256–264. [Google Scholar] [CrossRef]
- Borchmann, P.; Engert, A. The past what we have learned in the last decade. In Hematology 2010, the American Society of Hematology Education Program Book; American Society of Hematology: Suite, DC, USA, 2010; Volume 2010, pp. 101–107. [Google Scholar]
- Hodgson, D.C.; Pintilie, M.; Gitterman, L.; DeWitt, B.; Buckley, C.A.; Ahmed, S.; Smith, K.; Schwartz, A.; Tsang, R.W.; Crump, M.; et al. Fertility among female Hodgkin lymphoma survivors attempting pregnancy following ABVD chemotherapy. Hematol Oncol. 2007, 25, 11–15. [Google Scholar] [CrossRef]
- Behringer, K.; Breuer, K.; Reineke, T.; May, M.; Nogova, L.; Klimm, B.; Schmitz, T.; Wildt, L.; Diehl, V.; Engert, A. Secondary amenorrhea after Hodgkin’s lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: A report from Germany Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. 2005, 23, 7555–7564. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, M.L.; Huisbrink, J.; Hauptmann, M.; Kuenen, M.A.; Ouwens, G.M.; van Veer, M.B.; Aleman, B.M.; van Leeuwen, F.E. Treatment-related risk factors for premature menopause following Hodgkin lymphoma. Blood 2008, 111, 101–108. [Google Scholar] [CrossRef] [PubMed]







| Author/Year | Chemo Side Effects | HL Diagnosed Subtype | Treatment Received | HL Presenting Symptoms | Fetal Complication |
|---|---|---|---|---|---|
| Buchholz, M. L. et al., 2018 [4] | Fever | Nodular sclerosing | R-CHOEP (rituximab, cyclophosphamide, doxorubicin, vincristine, etoposide, prednisolone) | Dry cough, dyspnea, facial edema with hyperemia, night sweats, hepatosplenomegaly | None |
| Maggen, C. et al., 2019 [5] | Preterm contractions or preterm rupture of membrane | Nodular sclerosing | Standard ABVD (doxorubicin, bleomycin, vinblastine, dacarbazone) | Dry cough, dyspnea, night sweats | None |
| De Sanctis, V. et al., 2012 [6] | Fever | Nodular sclerosing | ABVD (Doxorubucin hydrochloride (Adriamycin), Bleomycin sulfate, Vinblastin sulfate, and Dacarbazin | Dry cough, night sweats, fatigue | None |
| Aviles, A. et al., 2018 [7] | Fever | Nodular sclerosing | ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastin sulfate, and Dacarbazin | Fatigue, Night sweats, dyspnea | None |
| Dunleavy, K. et al., 2020 [14] | Fever | Lymphocyte -rich | Total of 6-cycle of R-CHOP (Rituximab, cyclophosphamide, doxorubicin hydrochloride, Vincristine (Oncovin), Prednisone) | Chest pain, dyspnea, cramps in right calf | None |
| Bachanova, V. et al., 2013 [15] | Fever | Lymphocyte -rich | R-CHOP (Ritoximab, Cyclophosphamide, Doxorubicin hydrochloride, Vincristine (Oncovin), Prednisone) | Dyspnea, night sweats, dry cough | None |
| Eyre, T. A. et al., 2015 [8] | Fever, fatigue | Nodular sclerosing | ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastin sulfate, Dacarbazin) with single vinblastine in first trimester | Dyspnea, night sweats, fatigue | None |
| Gurevich-Shapiro, A. et al., 2019 [9] | Fatigue, fever, anorexia | Nodular sclerosing | R-CHOP (Ritoximab, Cyclophosphamide, Doxorubicin hydrochloride, Vincristine (Oncovin), Prdniisone) | Night sweats, fatigue, dyspnea, dry cough | None |
| Cotteret, C. et al., 2020 [16] | Fatigue, fever | Lymphocyte-rich | 2-cycle Standard ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastine sulfate, Dacarbazin) with 25 mg/m2 doxorubicin per cycle | Night sweats, dry cough, fatigue, dyspnea | Left cardiac dysfunction on day-4 after birth with high level of troponin which later resolved at 1 month of life. |
| Moshe, Y. et al., 2017 [10] | Fever, anorexia, nausea | Nodular sclerosing | ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastine sulfate, Dacarbazin) | Dry cough, fatigue, dyspnea, night sweats | None |
| El-Messidi, A. et al., 2015 [11] | Preterm birth, needed immediate post-partum blood transfusion, venous thromboembolism | Nodular sclerosing | ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastine sulfate, Dacarbazin) | Dry cough, night sweats, fatigue | None |
| Aisner, J. et al., 1993 [12] | None | Nodular sclerosing | R-CHOP (Ritoximab, Cyclophosphamide, Doxorubicin hydrochloridee, Vincristin (Oncovin), Prednisone | Night sweats, fatigue | None |
| Iriyama, N. et al., 2011 [19] | None | Stage-4 classical HL | Total of 6-cycle of ABVD (Doxorubucin hydrochloride, Bleomycin sulfate, Vinblastine sulfate, Dacarbazin) | Dry cough, fatigue, night sweats | None |
| Lishner, M. et al., 1992 [17] | None | Lymphocyte-rich | ABVD (Doxorubucin hydrochloride (Adriamycin), Bleomycin, Vinblastine sulfate, Dacarbazin) | Night sweats, dry cough, dyspnea | None |
| Anselmo, A. P et al., 1999 [18] | Anorexia, fever | Lymphocyte-rich | ABVD (Doxorubucin hydrochlorid (Adriamycin), Bleomycin sulfate, Vinblastine sulfate, Dacarbazin) | Dry cough, night sweats, weight loss, dyspnea | None |
| Korkontzelos, I. et al., 2005 [13] | None | Nodular sclerosing | Conservative/symptomatic treatment. Chemotherapy was given post-natal. | Dry cough, night sweats, fatigue | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delzotto, J.; Naqvi, T.S.; Opara, N.U.; Delzotto, A.; Morgan, A. Unusual Presentation of Hodgkin’s Lymphoma in Pregnancy: A Case Report and Systematic Review of Literature. Hematol. Rep. 2022, 14, 322-334. https://doi.org/10.3390/hematolrep14040046
Delzotto J, Naqvi TS, Opara NU, Delzotto A, Morgan A. Unusual Presentation of Hodgkin’s Lymphoma in Pregnancy: A Case Report and Systematic Review of Literature. Hematology Reports. 2022; 14(4):322-334. https://doi.org/10.3390/hematolrep14040046
Chicago/Turabian StyleDelzotto, Joseph, Tahira. S. Naqvi, Nnennaya. U. Opara, Anthony Delzotto, and Andrew Morgan. 2022. "Unusual Presentation of Hodgkin’s Lymphoma in Pregnancy: A Case Report and Systematic Review of Literature" Hematology Reports 14, no. 4: 322-334. https://doi.org/10.3390/hematolrep14040046