Biochemical Characteristics and Elemental Composition Peculiarities of Rheum tataricum L. in Semi-Desert Conditions and of European Garden Rhubarb
Abstract
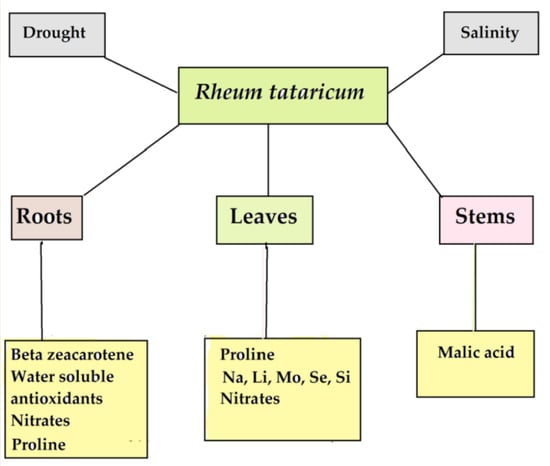
:1. Introduction
2. Materials and Methods
2.1. Place of Sampling
2.2. Mineral Composition
2.3. Total Polyphenols (TP)
2.4. Antioxidant Activity (AOA)
2.5. Carotenoids
2.6. Proline
2.7. Organic Acids
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Status of Plants
3.2. Proline
3.3. Carotenoids
3.4. Nitrates and Total Dissolved Solids
3.5. Organic Acids
3.6. Mineral Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Huang, Y.; Cai, L.Q.; Zhu, J.; Duan, Q.; Duan, Y.; Imperato-McGinley, J. The Chinese medicinal herbal formula ZYD88 inhibits cell growth and promotes cell apoptosis in prostatic tumor cells. Oncol. Rep. 2003, 10, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Chen, D.C. Effect of Rhubarb on Gastrointestinal Dysfunction in Critically Ill Patients: A Retrospective Study Based on Propensity Score Matching. Chin. Med. J. 2018, 131, 1142–1150. [Google Scholar] [CrossRef]
- Chen, D.; Wang, L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis. Chin. J. Traumatol. 2009, 12, 365–369. [Google Scholar]
- Chen, D.C.; Ma, L.Q.; Liu, S.Z. Effects of rhubarb on intestinal flora and bacterial translocation in rats with sepsis. Chin. Crit. Care Med. 2009, 21, 17–20. [Google Scholar]
- Kolodziejczyk-Czepas, J.; Czepas, J. Rhaponticin as an anti-inflammatory component of rhubarb: A mini review of the current state of the art and prospects for future research. Phytochem. Rev. 2019, 18, 1375–1386. [Google Scholar] [CrossRef]
- Tian, S.L.; Yang, Y.; Liu, X.L.; Xu, Q.B. Emodin Attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med. Sci. Monitor. 2018, 24, e937532. [Google Scholar] [CrossRef]
- Liudvytska, O.; Kolodziejczyk-Czepas, J. A Review on Rhubarb-Derived Substances as Modulators of Cardiovascular Risk Factors—A Special Emphasis on Anti-Obesity Action. Nutrients 2022, 14, 2053. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Katzir, G.; Ne’eman, G. Self-irrigation in the desert rhubarb Rheum palaestinum—A response to Khammash. Plant Ecol. Evol. 2017, 150, 109–111. [Google Scholar] [CrossRef]
- Chuikov, J.S. Bogdinsko-Baskunchack Nature Reserve Complex and Its Protection; Proceedings of the Bogdinsko-Baskunchak Nature Reserve: Akhtubinsk, Russia, 1998. (In Russian) [Google Scholar]
- Volobaeva, O.V. Bogdinsko-Baskunchak Nature Reserve Flora. Ph.D. Thesis, Bashkir State University, Ufa, Russia, 2021. (In Russian). [Google Scholar]
- Wulfsohn, D. Sampling Techniques for Plants and Soil; Landbauforschung Völkenrode, University of Copenhagen, Denmark, 2010, Special Issue 340, pp. 3–30.
- Kidin, V.V.; Derugin, I.P.; Kobzarenko, V.I. Workshop on Agrochemistry; Kolos: Moscow, Russia, 2007. (In Russian) [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.M.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020. (In Russian) [Google Scholar]
- Quertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef]
- Guidance on Methods of Quality Control and Safety of Biologically Active Food Supplements P 4.1.1672-03; Organic Acids Determination, 2004; Ministry of Health of Russia: Moscow, Russia, 2004; pp. 109–111. (In Russian)
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT-Food Sci. Technol. 2020, 118, 108775. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2021, 20, 589–607. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kharchenko, V.A.; Caruso, G. Selenium. Prospects of functional food production with high antioxidant activity. In Plant Antioxidants and Health; Reference Series in Phytochemistry; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Aichner, D.; Ganzera, M. Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta 2015, 144, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Kashiwada, Y.; Nanaka, G.I.; Nishioka, I. Tannins and related compounds. XLVIII. Rhubarb (7). Isolation and characterization of new dimeric and trimeric procyanidins. Chem. Pharmaceut. Bull. 1986, 34, 4089–4091. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Nanaka, G.I.; Nishioka, I. Chromone glucosides from rhubarb. Phytochemistry 1990, 29, 1007–1009. [Google Scholar] [CrossRef]
- Yagi, A.; Koizumi, Y.; Nishioka, I. Studies on rhubarb (Rheim Rhizoma). I—Stilbene derivatives from “Dodaioo” (Chinese inferior rhubarb). Jap. J. Pharmacol. 1971, 25, 52–54. [Google Scholar]
- Ge, Y.; Sun, M.; Salomé Abarca, L.F.; Wang, M.; · Choi, Y.H. Investigation of species and environmental effects on rhubarb roots metabolome using 1 H NMR combined with high performance thin layer chromatography. Metabolomics 2018, 14, 137. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.; Hashem, A.; Hashem, A.; Allah, E.; Gucel, S.; Tran, L.-S. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Petrusa, L.M.; Winicov, I. Proline status in salt tolerant and salt sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997, 35, 303–310. [Google Scholar]
- Fougère, F.; Le Rudulier, D.; Streeter, J.G. Effects of salt stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol. 1991, 96, 1228–1236. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Lado, J.; Alós, E.; Alquezar, B.; Dery, O.; Hirschberg, J.; Zacarias, L. A mutant allele of ζ-carotene isomerase (Z-ISO) is associated with the yellow pigmentation of the “Pinalate” sweet orange mutant and reveals new insights into its role in fruit carotenogenesis. BMC Plant Biol. 2019, 19, 465. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Zhang, G.-B.; Meng, S.; Gong, J.-M. The Expected and Unexpected Roles of Nitrate Transporters in Plant Abiotic Stress Resistance and Their Regulation. Int. J. Mol. Sci. 2018, 19, 3535. [Google Scholar] [CrossRef]
- Panchal, P.; Miller, A.J.; Giri, J. Organic acids: Versatile stress-response roles in plants. J. Exp. Bot. 2021, 72, 4038–4052. [Google Scholar] [CrossRef]
- Strobel, B.W.; Kristensen, F.; Hansen, H.C.B. Oxalate distribution in soils under rhubarb (Rheum rhaponticum). Int. J. Environ. Anal. Chem. 2004, 84, 909–917. [Google Scholar] [CrossRef]
- Fernando, D.R.; Mizuno, T.; Woodrow, I.E.; Baker, A.J.; Collins, R.N. Characterization of foliar manganese (Mn) in Mn (hyper)accumulators using X-ray absorption spectroscopy. New Phytol. 2010, 188, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hana, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Lombi, E.; van der Ent, A.; Wang, P.; Laird, J.S.; Moore, K.L.; Persson, D.P.; Husted, S. Methods to Visualize Elements in Plants. Plant Physiol. 2020, 182, 1869–1882. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Jin, L.; Peng, R. Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress. Plants 2022, 11, 1351. [Google Scholar] [CrossRef]
- Phong Thu, T.T.; Yasui, H.; Yamakawa, T. Effects of salt stress on plant growth characteristics and mineral content in diverse rice genotypes. Soil Sci. Plant Nutr. 2017, 63, 264–273. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Z.; Cheng, J.; Lai, Y.; Wang, J.; Bao, Y.; Huang, J.; Zhang, H. QTL Analysis of Na+ and K+ Concentrations in Roots and Shoots under Different Levels of NaCl Stress in Rice (Oryza sativa L.). PLoS ONE 2012, 7, 51202. [Google Scholar] [CrossRef]
- Anderson, C.E. Lithium in Plants. In Lithium and Cell Physiology; Bach, R.O., Gallicchio, V.S., Eds.; Springer: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Abou Seeda, M.A.; Yassen, A.A.; Abou El-Nour, E.A.A.; Zaghlou, S.M. Importance of Molybdenum and it Diverse Role in Plant Physiology: A Review. Middle East J. Appl. Sci. 2020, 10, 228–249. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The Role of Silicon in Higher Plants under Salinity and Drought Stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Xu, X.B.; Hu, Y.H.; Han, W.H.; Yin, J.L.; Li, H.L.; Gong, H.J. Silicon improves salt tolerance by increasing root water uptake in Cucumis sativus L. Plant Cell Rep. 2015, 34, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Cheema, M.A.; Abbas, T.; Sher, A.; Ijaz, M.; Hussain, M. Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russ. J. Plant Physiol. 2017, 64, 341–348. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, N.; Qin, D.; Liu, S.; Jiang, S.; Xu, L.; Sun, Z.; Yan, D.; Hu, A. The synergistic effects of silicon and selenium on enhancing salt tolerance of maize plants Environ. Exp. Bot. 2021, 187, 104482. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Object | Root Pulp | Root Peel | Florets | Stems | Leaves |
|---|---|---|---|---|---|---|
| AOA (mg GAE) g−1 d.w. | R. tataricum | 157.0 ± 12.0 a | 148.0 ± 11.0 a | 54.0 ± 3.0 d | 73.0 ± 4.0 bc | 83.0 ± 5.0 b |
| Zaryanka cv | 152.0 ± 20.0 a | 150.0 ± 13.0 a | 58.9 ± 2.5 cd | 51.5 ± 2.1 d | 68.3 ± 6.1 c | |
| TP (mg GAE) g−1 d.w. | R. tataricum | 25.1 ± 2.2 a | 24.6 ± 2.1 a | 23.6 ± 2.0 a | 23.0 ± 2.1 ab | 24.0 ± 2.1 a |
| Zaryanka cv | 24.9 ± 2.0 a | 19.5 ± 1.7 a | 21.8 ± 1.7 ab | 21.7 ± 1.9 ab | 21.3 ± 1.8 ab |
| Tissue | R. tataricum | Rhubarb, cv. Zaryanka |
|---|---|---|
| Root Peel | 54.1 ± 4.0 b | 26.0 ± 2.1 d |
| Root Pulp | 55.2 ± 4.1 b | 42.1 ± 3.0 c |
| Stems | 27.1 ± 2.1 d | 78.2 ± 6.1 a |
| Leaves | 71.1 ± 6.2 a | 51.0 ± 4.1 b |
| Organic Acid | R. tataricum | Rhubarb cv. Zaryanka |
|---|---|---|
| Citric | 64.00 ± 9.60 a | 74.25 ± 11.10 a |
| Oxalic | 7.50 ± 1.12 b | 12.76 ± 1.91 a |
| Malic | 3.40 ± 0.50 a | 0.27 ± 0.04 b |
| Succinic | 2.60 ± 0.39 b | 8.99 ± 1.34 a |
| Tartaric | 0.50 ± 0.07 a | 0.12 ± 0.02 b |
| Total | 78.0 ± 11.7 a | 96.40 ± 14.50 a |
| Element | R. tataricum | Rhubarb cv. Zaryanka | ||
|---|---|---|---|---|
| Roots | Leaves | Roots | Leaves | |
| Macroelements | ||||
| Ca | 36,239 ± 3624 a | 6550 ± 655 c | 18,675 ± 1868 b | 6738 ± 674 c |
| K | 7029 ± 703 c | 29,212 ± 2921 a | 5730 ± 573 c | 17,583 ± 1758 b |
| Mg | 2052 ± 205 c | 3507 ± 351 b | 843 ± 84 d | 5795 ± 580 a |
| Na | 1841 ± 184 b | 27,298 ± 2730 a | 29.08 ± 2.91 d | 55.89 ± 5.59 c |
| P | 858 ± 86 c | 4244 ± 424 a | 1681 ± 168 b | 5117 ± 512 a |
| Al, As and heavy metals | ||||
| Al | 83.1 ± 8.3 b | 161.0 ± 16.0 a | 39.9 ± 3.9 c | 104.0 ± 10.0 b |
| As | 0.04 ± 0.01 c | 0.2 ± 0.02 a | 0.03 ± 0.004 c | 0.14 ± 0.01 b |
| Cd | 0.06 ± 0.01 c | 0.27 ± 0.03 a | 0.05 ± 0.01 c | 0.13 ± 0.01 b |
| Cr | 1.18 ± 0.12 b | 3.24 ± 0.32 a | 0.29 ± 0.03 d | 0.81 ± 0.09 c |
| Ni | 1.66 ± 0.17 b | 1.72 ± 0.17 b | 1.93 ± 0.19 b | 3.03 ± 0.30 a |
| Pb | 0.20 ± 0.02 c | 0.73 ± 0.09 a | 0.13 ± 0.01 d | 0.53 ± 0.06 b |
| Sr | 172.0 ± 17.0 a | 21.5 ± 2.1 c | 109.0 ± 11.0 b | 20.3 ± 2.0 c |
| V | 0.41 ± 0.05 b | 0.77 ± 0.09 a | 0.13 ± 0.02 c | 0.36 ± 0.04 b |
| Microelements | ||||
| B | 9.93 ± 0.99 b | 17.01 ± 1.70 a | 5.22 ± 0.52 c | 4.92 ± 0.49 c |
| Co | 0.12 ± 0.01 b | 0.43 ± 0.05 a | 0.05 ± 0.01 c | 0.34 ± 0.04 a |
| Cu | 1.96 ± 0.20 c | 7.75 ± 0.77 a | 2.89 ± 0.29 b | 8.71 ± 0.87 a |
| Fe | 189.0 ± 19.0 c | 429.0 ± 43.1 a | 82.1 ± 8.0 d | 269.0 ± 27.0 b |
| I | 0.45 ± 0.06 bc | 0.53 ± 0.06 b | 2.07 ± 0.21 a | 0.39 ± 0.04 c |
| Li | 0.29 ± 0.03 b | 3.99 ± 0.40 a | 0.06 ± 0.01 c | 0.24 ± 0.02 b |
| Mn | 16.6 ± 1.7 c | 116.0 ± 12.0 b | 12.8 ± 1.3 c | 194.0 ± 19.0 a |
| Mo | 0.13 ± 0.01 c | 1.36 ± 0.14 a | 1.02 ± 0.10 b | 0.95 ± 0.11 b |
| Se | 0.04 ± 0.01 bc | 0.34 ± 0.04 a | 0.03 ± 0.01 c | 0.05 ± 0.01 b |
| Si | 3.29 ± 0.33 bc | 23.65 ± 2.37 a | 2.80 ± 0.28 c | 3.67 ± 0.37 b |
| Sn | 0.010 ± 0.002c | 0.050 ± 0.007 b | 0.120 ± 0.015 a | 0.110 ± 0.013 a |
| Zn | 7.60 ± 0.81 d | 30.01 ± 3.01 b | 13.82 ± 1.41 c | 43.60 ± 4.40 a |
| Parameter | Soil Total Content of Elements | Soil Bioavailable Forms of Elements | R. tataricum CBA |
|---|---|---|---|
| Soil salinity | 4825 ± 450 | - | - |
| Fe | 284.3 ± 28.0 | 3.5 ± 0.1 | 0.66 |
| Mn | 47.4 ± 4.0 | 5.9 ± 0.5 | 0.35 |
| Pb | 0.28 ± 0.02 | - | 0.92 |
| Cd | 0.14 ± 0.01 | - | 0.42 |
| Cu | 4.95 ± 0.50 | 0.52 ± 0.05 | 0.40 |
| Zn | 10.04 ± 1.00 | 0.14 ± 0.01 | 0.85 |
| Sr | 10.60 ± 1.00 | - | 16.23 |
| Cr | 7.22 ± 0.70 | - | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubkina, N.; Kharchenko, V.; Bogachuk, M.; Koshevarov, A.; Sheshnitsan, S.; Kosheleva, O.; Pirogov, N.; Caruso, G. Biochemical Characteristics and Elemental Composition Peculiarities of Rheum tataricum L. in Semi-Desert Conditions and of European Garden Rhubarb. Int. J. Plant Biol. 2022, 13, 368-380. https://doi.org/10.3390/ijpb13030031
Golubkina N, Kharchenko V, Bogachuk M, Koshevarov A, Sheshnitsan S, Kosheleva O, Pirogov N, Caruso G. Biochemical Characteristics and Elemental Composition Peculiarities of Rheum tataricum L. in Semi-Desert Conditions and of European Garden Rhubarb. International Journal of Plant Biology. 2022; 13(3):368-380. https://doi.org/10.3390/ijpb13030031
Chicago/Turabian StyleGolubkina, Nadezhda, Viktor Kharchenko, Maria Bogachuk, Andrew Koshevarov, Sergey Sheshnitsan, Olga Kosheleva, Nikolay Pirogov, and Gianluca Caruso. 2022. "Biochemical Characteristics and Elemental Composition Peculiarities of Rheum tataricum L. in Semi-Desert Conditions and of European Garden Rhubarb" International Journal of Plant Biology 13, no. 3: 368-380. https://doi.org/10.3390/ijpb13030031