Fabrication of Quercetin-Functionalized Morpholine and Pyridine Motifs-Laden Silk Fibroin Nanofibers for Effective Wound Healing in Preclinical Study
Abstract
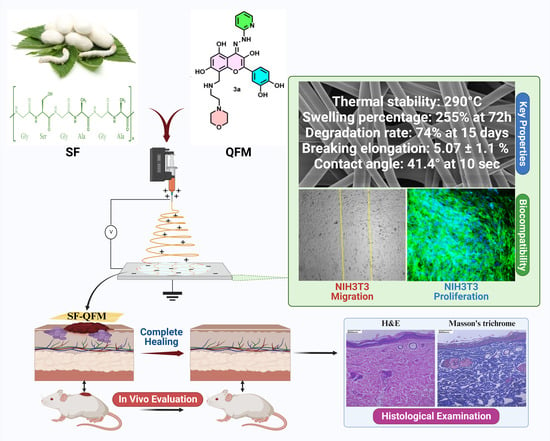
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for the Synthesis of Quercetin Pyridyl Hydrazone 3a (QFM):
2.3. Silk Fibroin Extraction from Bombyx mori
2.4. Fabrication of SF and SF-QFM Nanofibers Mat
2.5. NMR, FTIR, ESI-MS Measurement
2.6. Thermal Stability Analysis
2.7. Morphological Observation and Mechanical Property
2.8. Water Retention Capacity, Biodegradation Study, Contact Angle, and In Vitro Drug Release Study
2.9. Antimicrobial Activity, Antioxidant Assay, and In Vitro Cytocompatibility
2.10. Invitro Biocompatibility, Cell Adhesion, and Proliferation Studies
2.11. Scratch Assay
2.12. In Vivo Wound Healing Study and Histological Analysis
2.13. Statistical Analysis
3. Results
3.1. Chemistry
3.2. FTIR Analysis
3.3. Thermal Stability
3.4. Morphological Observation and Mechanical Properties
3.5. Water Retention Capacity, Biodegradation Study, Contact Angle, and In Vitro Drug Release Study
3.6. Antibacterial Activity, Antioxidant Assay, and In Vitro Cytocompatibility
3.7. In Vitro Biocompatibility, Cell Adhesion, and Proliferation Studies
3.8. Scratch Assay
3.9. In-Vivo Determination of the Wound Healing Activity
3.10. Histological Examination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The Human Skin Microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Dandona, R.; Kumar, G.A.; Gururaj, G.; James, S.; Chakma, J.K.; Thakur, J.S.; Srivastava, A.; Kumaresh, G.; Glenn, S.D.; Gupta, G.; et al. Mortality Due to Road Injuries in the States of India: The Global Burden of Disease Study 1990–2017. Lancet Public Health 2020, 5, e86–e98. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Phillips, C.J.; Harding, K. A Narrative Review of the Epidemiology and Economics of Chronic Wounds. Br. J. Dermatol. 2022, 187, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound Dressings: Current Advances and Future Directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Bolívar-Monsalve, E.J.; Alvarez, M.M.; Hosseini, S.; Espinosa-Hernandez, M.A.; Ceballos-González, C.F.; Sanchez-Dominguez, M.; Shin, S.R.; Cecen, B.; Hassan, S.; Di Maio, E.; et al. Engineering Bioactive Synthetic Polymers for Biomedical Applications: A Review with Emphasis on Tissue Engineering and Controlled Release. Mater. Adv. 2021, 2, 4447–4478. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization–The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin Promotes Cutaneous Wound Healing in Mice through Wnt/β-Catenin Signaling Pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.B.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.S.; Barreto, R.S.S. Wound Healing Properties of Flavonoids: A Systematic Review Highlighting the Mechanisms of Action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.T.A.; Teixeira, A.M.R.; Cassiano, C.J.M.; Sena, D.M.; Coutinho, H.D.M.; Menezes, I.R.A.; Figueredo, F.G.; Silva, L.E.; Toledo, T.A.; Bento, R.R.F. Modulation of the Antibiotic Activity against Multidrug Resistant Strains of 4-(Phenylsulfonyl) Morpholine. Saudi J. Biol. Sci. 2016, 23, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Qiu, H.Y.; Ma, J.T.; Yan, X.Q.; Gong, H.B.; Wang, Z.C.; Zhu, H.L. Dihydropyrazoles Containing Morpholine: Design, Synthesis and Bioassay Testing as Potent Antimicrobial Agents. RSC Adv. 2015, 5, 24997–25005. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Morpholine as Ubiquitous Pharmacophore in Medicinal Chemistry: Deep Insight into the Structure-Activity Relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef] [PubMed]
- Surendra Kumar, R.; Moydeen, M.; Al-Deyab, S.S.; Manilal, A.; Idhayadhulla, A. Synthesis of New Morpholine-Connected Pyrazolidine Derivatives and Their Antimicrobial, Antioxidant, and Cytotoxic Activities. Bioorg. Med. Chem. Lett. 2017, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Kumar, S.K.A.; Shah, S.K.; Kazi, S.; Sarkar, N.; Banerjee, S.; Dey, S. Pyridine: The Scaffolds with Significant Clinical Diversity. RSC Adv. 2022, 12, 15385–15406. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Burki, S.; El-Haj, B.M.; Shafiullah; Parveen, S.; Nadeem, H.Ş.; Nadeem, S.; Shah, M.R. Synthesis and Characterization of Pyridine-Based Organic Salts: Their Antibacterial, Antibiofilm and Wound Healing Activities. Bioorg. Chem. 2020, 100, 103937. [Google Scholar] [CrossRef] [PubMed]
- Allaka, T.R.; Katari, N.K. Synthesis of Pyridine Derivatives for Diverse Biological Activity Profiles: A Review. Recent Dev. Synth. Appl. Pyridines 2022, 605–625. [Google Scholar] [CrossRef]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Faheem; Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine- And Thiazole-Based Hydrazides with Promising Anti-Inflammatory and Antimicrobial Activities along with Their in Silico Studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef] [PubMed]
- Tahir, T.; Ashfaq, M.; Saleem, M.; Rafiq, M.; Shahzad, M.I.; Kotwica-Mojzych, K.; Mojzych, M. Pyridine Scaffolds, Phenols and Derivatives of Azo Moiety: Current Therapeutic Perspectives. Molecules 2021, 26, 4872. [Google Scholar] [CrossRef]
- Yao, X.; Zou, S.; Fan, S.; Niu, Q.; Zhang, Y. Bioinspired Silk Fibroin Materials: From Silk Building Blocks Extraction and Reconstruction to Advanced Biomedical Applications. Mater. Today Bio 2022, 16, 100381. [Google Scholar] [CrossRef]
- Sabarees, G.; Tamilarasi, G.P.; Velmurugan, V.; Alagarsamy, V.; Sibuh, B.Z.; Sikarwar, M.; Taneja, P.; Kumar, A.; Gupta, P.K. Emerging Trends in Silk Fibroin Based Nanofibers for Impaired Wound Healing. J. Drug Deliv. Sci. Technol. 2023, 79, 103994. [Google Scholar] [CrossRef]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk Fibroin and Silk-Based Biomaterial Derivatives for Ideal Wound Dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, L.; Wang, J.; Jin, M.; Tang, Q.; Chen, Z.; Cheng, Y.; Yang, R.; Zhao, G. Electrospun Nanofibers Promote Wound Healing: Theories, Techniques, and Perspectives. J. Mater. Chem. B 2021, 9, 3106–3130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, Z.; Han, Y.; Jiang, Q.; Zhan, C.; Zhang, K.; Li, Z.; Zhang, R. Structural Design and Environmental Applications of Electrospun Nanofibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106009. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Du, Y.; Zhang, J.; Zhang, H.; Guo, B. Structural and Functional Design of Electrospun Nanofibers for Hemostasis and Wound Healing. Adv. Fiber Mater. 2022, 4, 1027–1057. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Liang, B.; Yang, D.P.; Zeng, Y. Antibacterial Vitamin K3 Carnosine Peptide-Laden Silk Fibroin Electrospun Fibers for Improvement of Skin Wound Healing in Diabetic Rats. ACS Appl. Bio Mater. 2021, 4, 4769–4788. [Google Scholar] [CrossRef] [PubMed]
- Biagiotti, M.; Bassani, G.A.; Chiarini, A.; Vincoli, V.T.; Dal Prà, I.; Cosentino, C.; Alessandrino, A.; Taddei, P.; Freddi, G. Electrospun Silk Fibroin Scaffolds for Tissue Regeneration: Chemical, Structural, and Toxicological Implications of the Formic Acid-Silk Fibroin Interaction. Front. Bioeng. Biotechnol. 2022, 10, 833157. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Chen, S.H.; Lai, G.J. Preparation and Characterization of Biomimetic Silk Fibroin/Chitosan Composite Nanofibers by Electrospinning for Osteoblasts Culture. Nanoscale Res. Lett. 2012, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Che, L.; Ha, Y.; Ryu, W. Mechanically-Reinforced Electrospun Composite Silk Fibroin Nanofibers Containing Hydroxyapatite Nanoparticles. Mater. Sci. Eng. C 2014, 40, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.R.; Ju, H.W.; Lee, J.M.; Kim, D.-K.K.; Lee, O.J.; Moon, B.M.; Park, H.J.; Jeong, J.Y.; Yeon, Y.K.; Park, C.H. Three-Dimensional Electrospun Silk-Fibroin Nanofiber for Skin Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, C.H.; Lee, O.J.; Lee, J.M.; Kim, J.W.; Park, Y.H.; Ki, C.S. Preparation and in Vivo Degradation of Controlled Biodegradability of Electrospun Silk Fibroin Nanofiber Mats. J. Biomed. Mater. Res. Part A 2012, 100, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hsieh, M.J.; Chang, S.H.; Lin, Y.H.; Liu, S.J.; Lin, T.Y.; Hung, K.C.; Pang, J.H.S.; Juang, J.H. Enhancement of Diabetic Wound Repair Using Biodegradable Nanofibrous Metformin-Eluting Membranes: In Vitro and in Vivo. ACS Appl. Mater. Interfaces 2014, 6, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, D.; Lakra, R.; Korapatti, P.S.; Ramasamy, J.; Kiran, M.S. Nanoscaled Biodegradable Metal-Polymeric Three-Dimensional Framework for Endothelial Cell Patterning and Sustained Angiogenesis. ACS Biomater. Sci. Eng. 2019, 5, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Manthaisong, A.; Okonogi, S.; Tadtong, S.; Samee, W. Effects of Quercetin and Curcumin Combination on Antibacterial, Antioxidant, in Vitro Wound Healing and Migration of Human Dermal Fibroblast Cells. Int. J. Mol. Sci. 2022, 23, 142. [Google Scholar] [CrossRef]
- Sunil Richard, A.; Verma, R.S. Antioxidant α-Mangostin Coated Woven Polycaprolactone Nanofibrous Yarn Scaffold for Cardiac Tissue Repair. ACS Appl. Nano Mater. 2022, 5, 5075–5086. [Google Scholar] [CrossRef]
- Yunus Basha, R.; Sampath Kumar, T.S.; Selvaraj, R.; Doble, M. Silver Loaded Nanofibrous Curdlan Mat for Diabetic Wound Healing: An In Vitro and In Vivo Study. Macromol. Mater. Eng. 2018, 303, 1800234. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Zeng, Y. Fabrication of Vitamin K3-Carnosine Peptide-Loaded Spun Silk Fibroin Fibers/Collagen Bi-Layered Architecture for Bronchopleural Fistula Tissue Repair and Regeneration Applications. Biomater. Adv. 2022, 137, 212817. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, Y.; Rajinikanth, P.S.S.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin Loaded Polycaprolactone-/Polyvinyl Alcohol-Silk Fibroin Based Electrospun Nanofibrous Mat for Rapid Healing of Diabetic Wound: An in-Vitro and in-Vivo Studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, S.; Wang, L.; You, R.; Yan, S.; Zhang, Q.; Li, M. Bioactive Silk Fibroin Scaffold with Nanoarchitecture for Wound Healing. Compos. Part B Eng. 2021, 224, 109165. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.G.; Lee, B.T. Curcumin Incorporation into an Oxidized Cellulose Nanofiber-Polyvinyl Alcohol Hydrogel System Promotes Wound Healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; O’Connor, B.B.; Parker, K.K. Soy Protein/Cellulose Nanofiber Scaffolds Mimicking Skin Extracellular Matrix for Enhanced Wound Healing. Adv. Healthc. Mater. 2018, 7, 1701175. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, B.; Lu, X.B.; Zhang, Z.J.; Li, Z.; Li, W.L.; Xiong, A.B.; Deng, L.; Tan, M.Y.; Huang, Y.C. Grafting of Mesenchymal Stem Cell-Seeded Small Intestinal Submucosa to Repair the Deep Partial-Thickness Burns. Connect. Tissue Res. 2016, 57, 388–397. [Google Scholar] [CrossRef] [PubMed]










| Sample | Mean Tensile Strength (Mpa) | Mean Extension at Maximum Load (mm) | Mean Elongation at Break (%) | Mean Elongation at Break (mm) |
|---|---|---|---|---|
| SF | 9.47 ± 0.3 | 2.41 ± 0.6 | 3.75 ± 1.2 | 5.68 ± 2.8 |
| SF-QFM | 10.38 ± 0.7 | 3.64 ± 0.3 | 5.07 ± 1.1 | 6.32 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabarees, G.; Velmurugan, V.; Gouthaman, S.; Solomon, V.R.; Kandhasamy, S. Fabrication of Quercetin-Functionalized Morpholine and Pyridine Motifs-Laden Silk Fibroin Nanofibers for Effective Wound Healing in Preclinical Study. Pharmaceutics 2024, 16, 462. https://doi.org/10.3390/pharmaceutics16040462
Sabarees G, Velmurugan V, Gouthaman S, Solomon VR, Kandhasamy S. Fabrication of Quercetin-Functionalized Morpholine and Pyridine Motifs-Laden Silk Fibroin Nanofibers for Effective Wound Healing in Preclinical Study. Pharmaceutics. 2024; 16(4):462. https://doi.org/10.3390/pharmaceutics16040462
Chicago/Turabian StyleSabarees, Govindaraj, Vadivel Velmurugan, Siddan Gouthaman, Viswas Raja Solomon, and Subramani Kandhasamy. 2024. "Fabrication of Quercetin-Functionalized Morpholine and Pyridine Motifs-Laden Silk Fibroin Nanofibers for Effective Wound Healing in Preclinical Study" Pharmaceutics 16, no. 4: 462. https://doi.org/10.3390/pharmaceutics16040462