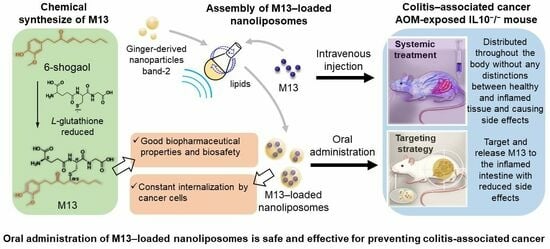
Prevention of Colitis-Associated Cancer via Oral Administration of M13-Loaded Lipid Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fabrication of Nano-Liposomes (NL)
2.3. Characterization of NL
2.4. Cell Uptake Study
2.5. In Vivo Cancer Prevention Test on AOM-Exposed IL10−/− Mouse Model of CAC
2.5.1. Animal
2.5.2. Treatment Groups
2.5.3. Bodyweight, Fecal Lipocalin Measurement, and Colonoscopy
2.5.4. Intestinal Microbiota
2.5.5. Tumor Counting, Colon Length, and Spleen Weight
2.6. Intratumoral Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Assembly and Characterization of M13–NL, NL, and DiL-Labeled NL
3.2. NL Is Continuously Internalized by Cancerous Epithelial Cells
3.3. Oral Administration of M13–NL Prevents Colonic Tumorigenesis in AOM-Exposed IL10−/− Mice
3.4. Oral Administration of M13–NL Regulates Colonic Microbiota in the AOM-Exposed IL10−/− Mice
3.5. Oral Administration of M13–NL Increased Intratumoral Microbiota Species in the AOM-Exposed IL10−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maryńczak, K.; Włodarczyk, J.; Sabatowska, Z.; Dziki, A.; Dziki, Ł.; Włodarczyk, M. Colitis-Associated Colorectal Cancer in Patients with Inflammatory Bowel Diseases in a Tertiary Referral Center: A Propensity Score Matching Analysis. J. Clin. Med. 2022, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Zinger, A.; Ben-Neriah, Y. Inflammatory networks underlying colorectal cancer. Nat. Immunol. 2016, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Luo, C.; Zhang, H. Early detection of ulcerative colitis-associated colorectal cancer. Gastroenterol. Rep. 2018, 6, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Cagan, A.; Cai, T.; Gainer, V.S.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; Kohane, I.; Liao, K.P. Colonoscopy Is Associated with a Reduced Risk for Colon Cancer and Mortality in Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2015, 13, 322–329.e1. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Muscat, J. Potential Role of Non-Steroidal Anti-Inflammatory Drugs in Colorectal Cancer Chemoprevention for Inflammatory Bowel Disease: An Umbrella Review. Cancers 2023, 15, 1102. [Google Scholar] [CrossRef]
- Carter, M.J.; Lobo, A.J.; Travis, S.P.L. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004, 53, v1–v16. [Google Scholar] [CrossRef]
- Zhao, L.N.; Li, J.Y.; Yu, T.; Chen, G.C.; Yuan, Y.H.; Chen, Q.K. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: An updated meta-analysis. PLoS ONE 2014, 9, e94208. [Google Scholar] [CrossRef]
- Del Sordo, R.; Lougaris, V.; Bassotti, G.; Armuzzi, A.; Villanacci, V. Therapeutic agents affecting the immune system and drug-induced inflammatory bowel disease (IBD): A review on etiological and pathogenetic aspects. Clin. Immunol. 2022, 234, 108916. [Google Scholar] [CrossRef]
- Wang, Y.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2016, 2016, CD000544. [Google Scholar] [CrossRef]
- Davis, A.; Robson, J. The dangers of NSAIDs: Look both ways. Br. J. Gen. Pract. 2016, 66, 172–173. [Google Scholar] [CrossRef]
- Drożdżal, S.; Lechowicz, K.; Szostak, B.; Rosik, J.; Kotfis, K.; Machoy-Mokrzyńska, A.; Białecka, M.; Ciechanowski, K.; Gawrońska-Szklarz, B. Kidney damage from nonsteroidal anti-inflammatory drugs-Myth or truth? Review of selected literature. Pharmacol. Res. Perspect. 2021, 9, e00817. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.F.; Zhu, L.L.; Chen, M.; Xu, H.M.; Wang, H.F.; Feng, X.Q.; Zhu, X.P.; Zhou, Q. The optimal choice of medication administration route regarding intravenous, intramuscular, and subcutaneous injection. Patient Prefer. Adherence 2015, 9, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.; Flores, S.R.L.; Woloshun, R.R.; Yang, C.; Yin, L.; Xiang, P.; Xu, X.; Garrick, M.D.; Vidyasagar, S.; et al. Oral Gavage of Ginger Nanoparticle-Derived Lipid Vectors Carrying Dmt1 siRNA Blunts Iron Loading in Murine Hereditary Hemochromatosis. Mol. Ther. 2019, 27, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Merlin, D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, M.; Lama, S.; Wang, L.; Merlin, D. Natural-lipid nanoparticle-based therapeutic approach to deliver 6-shogaol and its metabolites M2 and M13 to the colon to treat ulcerative colitis. J. Control. Release 2020, 323, 293–310. [Google Scholar] [CrossRef]
- Yang, C.; Sung, J.; Long, D.; Alghoul, Z.; Merlin, D. Prevention of Ulcerative Colitis by Autologous Metabolite Transfer from Colitogenic Microbiota Treated with Lipid Nanoparticles Encapsulating an Anti-Inflammatory Drug Candidate. Pharmaceutics 2022, 14, 1233. [Google Scholar] [CrossRef]
- Rothemich, A.; Arthur, J.C. The Azoxymethane/Il10−/− Model of Colitis-Associated Cancer (CAC). In Mouse Models of Innate Immunity; Springer: Berlin, Germany, 2019; pp. 215–225. [Google Scholar]
- Reddy, B.S. Animal Models for Colon Cancer Chemoprevention. In Encyclopedia of Cancer, 2nd ed.; Bertino, J.R., Ed.; Academic Press: New York, NY, USA, 2002; pp. 49–55. [Google Scholar]
- Zhu, Y.; Warin, R.F.; Soroka, D.N.; Chen, H.; Sang, S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation. PLoS ONE 2013, 8, e54677. [Google Scholar] [CrossRef]
- Dai, L.; Li, S.; Hao, Q.; Zhou, R.; Zhou, H.; Lei, W.; Kang, H.; Wu, H.; Li, Y.; Ma, X. Low-density lipoprotein: A versatile nanoscale platform for targeted delivery. Nanoscale Adv. 2023, 5, 1011–1022. [Google Scholar] [CrossRef]
- Uronis, J.M.; Mühlbauer, M.; Herfarth, H.H.; Rubinas, T.C.; Jones, G.S.; Jobin, C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 2009, 4, e6026. [Google Scholar] [CrossRef]
- McFadden, R.-M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Brückner, M.; Lenz, P.; Nowacki, T.M.; Pott, F.; Foell, D.; Bettenworth, D. Murine endoscopy for in vivo multimodal imaging of carcinogenesis and assessment of intestinal wound healing and inflammation. J. Vis. Exp. 2014, 26, 51875. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- De Robertis, M.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J. Carcinog. 2011, 10, 9. [Google Scholar]
- Long, D.; Alghoul, Z.; Sung, J.; Yang, C.; Merlin, D. Oral administration of M13-loaded nanoliposomes is safe and effective to treat colitis-associated cancer in mice. Expert. Opin. Drug Deliv. 2023, 1–20. [Google Scholar] [CrossRef]
- Gomes-Santos, A.C.; Moreira, T.G.; Castro-Junior, A.B.; Horta, B.C.; Lemos, L.; Cruz, D.N.; Guimarães, M.A.F.; Cara, D.C.; McCafferty, D.-M.; Faria, A.M.C. New Insights into the Immunological Changes in IL-10-Deficient Mice during the Course of Spontaneous Inflammation in the Gut Mucosa. Clin. Dev. Immunol. 2012, 2012, 560817. [Google Scholar] [CrossRef]
- Fang, C.Y.; Chen, J.S.; Hsu, B.M.; Hussain, B.; Rathod, J.; Lee, K.H. Colorectal Cancer Stage-Specific Fecal Bacterial Community Fingerprinting of the Taiwanese Population and Underpinning of Potential Taxonomic Biomarkers. Microorganisms 2021, 9, 1548. [Google Scholar] [CrossRef]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, D.; Alghoul, Z.; Sung, J.; Yang, C.; Merlin, D. Prevention of Colitis-Associated Cancer via Oral Administration of M13-Loaded Lipid Nanoparticles. Pharmaceutics 2023, 15, 2331. https://doi.org/10.3390/pharmaceutics15092331
Long D, Alghoul Z, Sung J, Yang C, Merlin D. Prevention of Colitis-Associated Cancer via Oral Administration of M13-Loaded Lipid Nanoparticles. Pharmaceutics. 2023; 15(9):2331. https://doi.org/10.3390/pharmaceutics15092331
Chicago/Turabian StyleLong, Dingpei, Zahra Alghoul, Junsik Sung, Chunhua Yang, and Didier Merlin. 2023. "Prevention of Colitis-Associated Cancer via Oral Administration of M13-Loaded Lipid Nanoparticles" Pharmaceutics 15, no. 9: 2331. https://doi.org/10.3390/pharmaceutics15092331