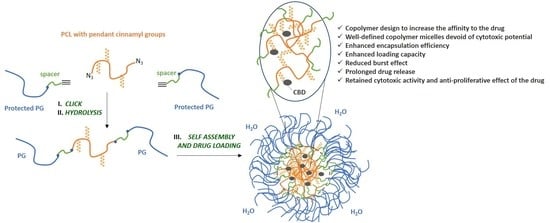
Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyglycidol-Block-Poly(Propylene Oxide)-Block-Poly(α-Cinnamyl-ɛ-Caprolactone-co-ɛ-Caprolactone)-Block-Poly(Propylene Oxide)-Block-Polyglycidol Block Copolymer
2.3. Preparation of Polymeric Micelles
2.4. Loading of CBD
2.5. Proton Nuclear Magnetic Resonance (1H-NMR)
2.6. Size Exclusion Chromatography (SEC)
2.7. Transmission Electron Microscopy (TEM)
2.8. Atomic Force Microscopy (AFM)
2.9. Dynamic and Electrophoretic Light Scattering
2.10. Multiangle Dynamic and Static Light Scattering
2.11. Spectrophotometric Determination of the Critical Micelle Concentration (CMC)
2.12. Drug-Release Study
2.13. Cytotoxicity Assessment
2.14. Statistical Analysis
3. Results and Discussion
3.1. Copolymer Synthesis and Characterization
3.2. Preparation of Blank and CBD-Loaded Polymeric Micelles
3.3. In Vitro Drug Release and Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolas, J. Drug-Initiated Synthesis of Polymer Prodrugs: Combining Simplicity and Efficacy in Drug Delivery. Chem. Mater. 2016, 28, 1591–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Maheshwari, R.; Kiick, K.L. Polymer-Based Therapeutics. Macromolecules 2009, 42, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Li, C.; Wang, Z.; Liu, J. Factors affecting the stability of drug-loaded polymeric micelles and strategies for improvement. J. Nanoparticle Res. 2016, 18, 275. [Google Scholar] [CrossRef]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Chen, L.G.; Strasburg, S.H.; Bermudez, H. Micelle co-assembly in surfactant/ionic liquid mixtures. J. Colloid Interface Sci. 2016, 477, 40–45. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef]
- Deng, W.; Chen, J.; Kulkarnia, A.; Thompson, D.H. Poly(ethylene glycol)-poly(vinyl alcohol)-adamantanate: Synthesis and stimuli-responsive micelle properties. Soft Matter 2012, 8, 5843–5846. [Google Scholar] [CrossRef]
- Foster, J.C.; Akar, I.; Grocott, M.C.; Pearce, A.K.; Mathers, R.T.; O’Reilly, R.K. 100th Anniversary of Macromolecular Science Viewpoint: The Role of Hydrophobicity in Polymer Phenomena. ACS Macro Lett. 2020, 9, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, C.; Hu, G.H.; Wang, J.; Ma, J.H.; Liang, X.F.; Zheng, L.; Liu, H.Z. Effect of hydrophobicity inside PEO-PPO-PEO block copolymer micelles on the stabilization of gold nanoparticles: Experiments. Langmuir 2006, 22, 9704–9711. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Van Butsele, K.; Cajot, S.; Van Vlierberghe, S.; Dubruel, P.; Passirani, C.; Benoit, J.-P.; Jérôme, R.; Jérôme, C. pH-Responsive Flower-Type Micelles Formed by a Biotinylated Poly(2-vinylpyridine)-block-poly(ethylene oxide)-block-poly(ε-caprolactone) Triblock Copolymer. Adv. Funct. Mater. 2009, 19, 1416–1425. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tong, Y.W.; Yang, Y.Y. Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(d,l-lactide-co-glycolide) with varying compositions. Biomaterials 2005, 26, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Gómez-Mascaraque, L.G.; Aguilar, M.R.; Vázquez-Lasa, B.; San Román, J. Self-assembling polymer systems for advanced treatment of cancer and inflammation. Prog. Polym. Sci. 2016, 53, 207–248. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Werner Hutmacher, D. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, N.; Manahan, R.; Lew, Z.T.; Jonathan, Y.; Xu, J.; Boyer, C. Copolymers with Controlled Molecular Weight Distributions and Compositional Gradients through Flow Polymerization. Macromolecules 2018, 51, 4553–4563. [Google Scholar] [CrossRef]
- Iskin, B.; Yilmaz, G.; Yagci, Y. ABC type miktoarm star copolymers through combination of controlled polymerization techniques with thiol-ene and azide-alkyne click reactions. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2417–2422. [Google Scholar] [CrossRef]
- Atanasova, M.D.; Grancharov, G.; Petrov, P.D. Poly(ethylene oxide)-block-poly(α-cinnamyl-ε-caprolactone-co-ε-caprolactone) diblock copolymer nanocarriers for enhanced solubilization of caffeic acid phenethyl ester. J. Polym. Sci. 2021, 59, 251–260. [Google Scholar] [CrossRef]
- Kalinova, R.; Yordanov, Y.; Tzankov, B.; Tzankova, V.; Yoncheva, K.; Dimitrov, I. Cinnamyl modified polymer micelles as efficient carriers of caffeic acid phenethyl ester. React. Funct. Polym. 2020, 157, 104763. [Google Scholar] [CrossRef]
- Liu, S.; Kobayashi, S.; Nishimura, S.; Ueda, T.; Tanaka, M. Effect of pendant groups on the blood compatibility and hydration states of poly(2-oxazoline)s. J. Polym. Sci. 2021, 59, 2559. [Google Scholar] [CrossRef]
- Garg, S.M.; Vakili, M.R.; Lavasanifar, A. Polymeric micelles based on poly(ethylene oxide) and α-carbon substituted poly(ɛ-caprolactone): An in vitro study on the effect of core forming block on polymeric micellar stability, biocompatibility, and immunogenicity. Colloids Surf. B Biointerfaces 2015, 132, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Son, I.; Lee, Y.; Baek, J.; Park, M.; Han, D.; Min, S.K.; Lee, D.; Kim, B.-S. pH-Responsive Amphiphilic Polyether Micelles with Superior Stability for Smart Drug Delivery. Biomacromolecules 2021, 22, 2043–2056. [Google Scholar] [CrossRef]
- Grancharov, G.; Gancheva, V.; Kyulavska, M.; Momekova, D.; Momekov, G.; Petrov, P. Functional multilayered polymeric nanocarriers for delivery of mitochondrial targeted anticancer drug curcumin. Polymer 2016, 84, 27–37. [Google Scholar] [CrossRef]
- Hu, X.; Jing, X. Biodegradable amphiphilic polymer–drug conjugate micelles. Expert Opin. Drug Deliv. 2009, 6, 1079–1090. [Google Scholar] [CrossRef]
- Akter, S.; Clem, B.F.; Lee, H.J.; Chesney, J.; Bae, Y. Block Copolymer Micelles for Controlled Delivery of Glycolytic Enzyme Inhibitors. Pharm. Res. 2011, 29, 847–855. [Google Scholar] [CrossRef]
- Zhao, Y. Photocontrollable block copolymer micelles: What can we control? J. Mater. Chem. 2009, 19, 4887. [Google Scholar] [CrossRef]
- Lee, H.J.; Bae, Y. Brushed Block Copolymer Micelles with pH-Sensitive Pendant Groups for Controlled Drug Delivery. Pharm. Res. 2013, 30, 2077–2086. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Xiao, Y.; Mao, A.; Lang, M. Phenylboronic acid conjugated mPEG-b-PCL micelles as DOX carriers for enhanced drug encapsulation and controlled drug release. Eur. Polym. J. 2022, 173, 111235. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, M.; He, J.; Ni, P. Synthesis and characterization of amphiphilic poly(ɛ-caprolactone)-b-polyphosphoester diblock copolymers bearing multifunctional pendant groups. Polymer 2012, 53, 2854–2863. [Google Scholar] [CrossRef]
- Lee, C.C.; Su, Y.C.; Ko, T.P.; Lin, L.L.; Yang, C.Y.; Chang, S.S.; Roffler, S.R.; Wang, A.H. Structural basis of polyethylene glycol recognition by antibody. J. Biomed. Sci. 2020, 27, 12. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug. Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Lila, A.S.A.; Nawata, K.; Shimizu, T.; Ishida, T.; Kiwada, H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int. J. Pharm. 2013, 456, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Dimde, M.; Weise, C.; Pouyan, P.; Licha, K.; Schirner, M.; Haag, R. Polyglycerol for Half-Life Extension of Proteins—Alternative to PEGylation? Biomacromolecules 2021, 22, 1406–1416. [Google Scholar] [CrossRef]
- Maruyama, K.; Okuizumi, S.; Ishida, O.; Yamauchi, H.; Kikuchi, H.; Iwatsuru, M. Phosphatidyl polyglycerols prolong liposome circulation in vivo. Int. J. Pharm. 1994, 111, 103–107. [Google Scholar] [CrossRef]
- Dworak, A.; Walach, W.; Trzebicka, B. Cationic polymerization of glycidol. Polymer structure and polymerization mechanism. Macromol. Chem. Phys. 1995, 196, 1963–1970. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(ethylene glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Kainthan, R.K.; Janzen, J.; Levin, E.; Devine, D.V.; Brooks, D.E. Biocompatibility Testing of Branched and Linear Polyglycidol. Biomacromolecules 2006, 7, 703–709. [Google Scholar] [CrossRef]
- Taton, D.; Le Borgne, A.; Sepulchre, M.; Spassky, N. Synthesis of chiral and racemic functional polymers from glycidol and thioglycidol. Macromol. Chem. Phys. 1994, 195, 139–148. [Google Scholar] [CrossRef]
- Dworak, A.; Baran, G.; Trzebicka, B.; Wałach, W. Polyglycidolblock-poly(ethylene oxide)-block-polyglycidol: Synthesis and swelling properties. React. Funct. Polym. 1999, 42, 31–36. [Google Scholar] [CrossRef]
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef]
- Erberich, M.; Keul, H.; Möller, M. Polyglycidols with two orthogonal protective groups: Preparation, selective deprotection, and functionalization. Macromolecules 2007, 40, 3070–3079. [Google Scholar] [CrossRef]
- Toncheva-Moncheva, N.; Bakardzhiev, P.; Rangelov, S.; Trzebicka, B.; Forys, A.; Petrov, P.D. Linear Amphiphilic Polyglycidol/Poly(ε-caprolactone) Block Copolymers Prepared via “Click” Chemistry-Based Concept. Macromolecules 2019, 52, 3435–3447. [Google Scholar] [CrossRef]
- Bakardzhiev, P.; Toncheva-Moncheva, N.; Mladenova, K.; Petrova, S.; Videv, P.; Moskova-Doumanova, V.; Topouzova-Hristova, T.; Doumanov, J.; Rangelov, S. Assembly of amphiphilic nucleic acid-polymer conjugates into complex superaggregates: Preparation, properties, and in vitro performance. Eur. Polym. J. 2020, 131, 109692. [Google Scholar] [CrossRef]
- Maroon, J.; Bost, J. Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 2018, 9, 91. [Google Scholar] [CrossRef]
- Vlad, R.A.; Hancu, G.; Ciurba, A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Silasi, O.; Muntean, D.L. Cannabidiol—Therapeutic and legal aspects. Pharmazie 2020, 75, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; Stephens, G.J. Development of cannabidiol as a treatment for severe childhood epilepsies. Br. J. Pharmacol. 2020, 177, 5509–5517. [Google Scholar] [CrossRef] [PubMed]
- Grancharov, G.; Atanasova, M.D.; Aluani, D.; Yoncheva, K.; Tzankova, V.; Trusheva, B.; Forys, A.; Trzebicka, B.; Petrov, P.D. Functional block copolymers bearing pendant cinnamyl groups for enhanced solubilization of caffeic acid phenethyl ester. Polym. J. 2020, 52, 435–447. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Varma, R.S. Solvent-free tetrahydropyranylation (THP) of alcohols and phenols and their regeneration by catalytic aluminum chloride hexahydrate. Tetrahedron Lett. 2002, 43, 1143–1146. [Google Scholar] [CrossRef]
- Dimitrov, P.; Rangelov, S.; Dworak, A.; Haraguchi, N.; Hirao, A.; Tsvetanov, C.B. Triblock and radial star-block copolymers comprised of poly(ethoxyethyl glycidyl ether), polyglycidol, poly(propylene oxide) and polystyrene obtained by anionic polymerization initiated by Cs initiators. Macromol. Symp. 2004, 215, 127–140. [Google Scholar] [CrossRef]
- Harris, J.; Roos, C.; Djalali, R.; Rheingans, O.; Maskos, M.; Schmidt, M. Application of the negative staining technique to both aqueous and organic solvent solutions of polymer particles. Micron 1999, 30, 289–298. [Google Scholar] [CrossRef]
- Provencher, S.W. Inverse Problems in Polymer Characterization: Direct Analysis of Polydispersity with Photon Correlation Spectroscopy. Macromol. Chem. 1979, 180, 201–209. [Google Scholar] [CrossRef]
- D’Souza, S.S.; DeLuca, P.P. Methods to Assess in Vitro Drug Release from Injectable Polymeric Particulate Systems. Pharm. Res. 2006, 23, 460–474. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Konstantinov, S.; Eibl, H.; Berger, M. BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–374. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers in Aqueous Solutions: Thermodynamics of Copolymer Association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Scherlund, M.; Brodin, A.; Malmsten, M. Micellization and gelation in block copolymer systems containing local anesthetics. Int. J. Pharm. 2000, 211, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Halacheva, S.; Rangelov, S.; Tsvetanov, C. Synthesis of Polyglycidol-Based Analogues to Pluronic L121–F127 Copolymers. Self-Assembly, Thermodynamics, Turbidimetric and Rheological Studies. Macromolecules 2008, 41, 7699–7705. [Google Scholar] [CrossRef]
- Burchard, W. Static and Dynamic Light Scattering from Branched Polymers and Biopolymers. In Light Scattering from Polymers. Adv. Polym. Sci. 1983, 48, 1. [Google Scholar] [CrossRef]
- Burchard, W. Light Scattering: Principles and Development; Brown, W., Ed.; Clarendon Press: Oxford, UK, 1996; p. 439. [Google Scholar]
- Rangelov, S.; Almgren, M.; Halacheva, S.; Tsvetanov, C. Polyglycidol-Based Analogues to Pluronic® Copolymers. Light Scattering and Cryogenic Transmission Electron Microscopy Studies. J. Phys. Chem. C 2007, 111, 13185–13191. [Google Scholar] [CrossRef]
- Rangelov, S.; Halacheva, S.; Garamus, V.; Almgren, M. Structural Polymorphism Exhibited by Polyglycidol-Based Analogues to Pluronic Copolymers in Aqueous Solution. Macromolecules 2008, 41, 8885–8894. [Google Scholar] [CrossRef]
- Flory, P.J. Principle of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. J. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 1999; p. VII-675-713. [Google Scholar]
- Gupta, A.; Costa, A.P.; Xu, X.; Lee, S.-L.; Cruz, C.N.; Bao, Q.; Burgess, D.J. Formulation and characterization of curcumin loaded polymeric micelles produced via continuous processing. Int. J. Pharm. 2020, 583, 119340. [Google Scholar] [CrossRef]
- Bromberg, L. Polymeric micelles in oral chemotherapy. J. Control. Release 2008, 128, 99–112. [Google Scholar] [CrossRef]
- Tonk, S.; Rápó, E. Linear and Nonlinear Regression Analysis for the Adsorption of Remazol Dye by Romanian Brewery Waste By-Product, Saccharomyces cerevisiae. Int. J. Mol. Sci. 2022, 23, 11827. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Chan, J., W.; Uhrich, K.E. Drug loading and release kinetics in polymeric micelles: Comparing dynamic versus unimolecular sugar-based micelles for controlled release. J. Bioact. Compat. Polym. 2016, 31, 227–241. [Google Scholar] [CrossRef]











| Copolymer | Mn a (g·mol−1) | Mw b (g·mol−1) | Mw/Mn b |
|---|---|---|---|
| PEEGE45-b-PCL35-b-PEEGE45 | 17,400 | 15,300 | 1.8 |
| PG45-b-PCL35-b-PG45 | 11,000 | - | - |
| PEEGE50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PEEGE50 | 20,100 | 15,000 | 1.3 |
| PG50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PG50 | 13,200 | - | - |
| Copolymer | Rh90 (nm) | ζ Potential (mV) | EE (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Blank | Loaded Prot. A | Loaded Prot. B | Blank | Loaded Prot. A | Loaded Prot. B | Loaded Prot. A | Loaded Prot. B | |
| PG45-b-PCL35-b-PG45 | 52.0 ± 1.7 | 56.0 ± 2.7 | 50.0 ± 3.8 | 2.94 ± 1.97 | −2.61 ± 2.10 | 2.90 ± 2.40 | 91.0 | 82.0 |
| PG50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PG50 | 60.0 ± 1.6 | 51.0 ± 1.8 | 57.0 ± 1.4 | −5.90 ± 1.23 | −6.61 ± 3.10 | 4.19 ± 2.50 | 95.0 | 92.0 |
| Rh (nm) | 10−6 × Mw (g·mol−1) | Rg (nm) | Rg/Rh | 106 × A2 mL·mol/g2 | ρ a (mg·mL−1) | Loading Capacity b | |
|---|---|---|---|---|---|---|---|
| Empty | 59.1 | 27.560 | 59.1 | 1.00 | −2.6 | 52.9 | n.a. |
| CBD-loaded | 53.8 | 26.970 | 54.2 | 1.03 | −2.8 | 68.7 | 8161 |
| Kinetic Model | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | R2 | Ko (mg·mL−1)/h | t1/2 (h) | R2 | KF (h−1) | t1/2 (h) | R2 | KH (mg·mL−1)/h0.5 | R2 | KKP (hn) | n |
| PG50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PG50:CBD (protocol A) | 0.746 | 1.148 | 0.043 | 0.808 | −0.007 | 99 | 0.943 | 7.467 | 0.978 | −0.845 | 0.366 |
| PG50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PG50:CBD (protocol B) | 0.782 | 1.017 | 0.049 | 0.707 | −0.006 | 115.5 | 0.946 | 6.341 | 0.970 | −0.692 | 0.261 |
| PG45-b-PCL35-b-PG45:CBD (protocol A) | 0.675 | 1.381 | 0.036 | 0.753 | −0.010 | 69.3 | 0.926 | 9.562 | 0.986 | −0.696 | 0.353 |
| PG45-b-PCL35-b-PG45:CBD (protocol B) | 0.722 | 1.495 | 0.033 | 0.886 | −0.016 | 43.31 | 0.957 | 9.955 | 0.977 | −0.615 | 0.315 |
| Sample | IC50 | |||
|---|---|---|---|---|
| HL-60 | HUT-78 | |||
| Protocol A | Protocol B | Protocol A | Protocol B | |
| PG45-b-PCL35-b-PG45:CBD | 2.33 | 2.29 | 5.26 | 5.17 |
| PG50-b-PPO4-b-[P(CyCL)4-co-(CL)40]-b-PPO4-b-PG50:CBD | 3.00 | 3.20 | 8.30 | 8.00 |
| Pure CBD | 2.00 | 7.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toncheva-Moncheva, N.; Dimitrov, E.; Grancharov, G.; Momekova, D.; Petrov, P.; Rangelov, S. Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol. Pharmaceutics 2023, 15, 2128. https://doi.org/10.3390/pharmaceutics15082128
Toncheva-Moncheva N, Dimitrov E, Grancharov G, Momekova D, Petrov P, Rangelov S. Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol. Pharmaceutics. 2023; 15(8):2128. https://doi.org/10.3390/pharmaceutics15082128
Chicago/Turabian StyleToncheva-Moncheva, Natalia, Erik Dimitrov, Georgi Grancharov, Denitsa Momekova, Petar Petrov, and Stanislav Rangelov. 2023. "Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol" Pharmaceutics 15, no. 8: 2128. https://doi.org/10.3390/pharmaceutics15082128